More Information
Submitted: 11 July 2019 | Approved: 22 July 2019 | Published: 23 July 2019
How to cite this article: Atanásio-Nhacumbe A, Sitoe CF. Prevalence and seasonal variations of eggs of gastrointestinal nematode parasites of goats from smallholder farms in Mozambique. Insights Vet Sci. 2019; 3: 023-029. doi: 10.29328/journal.ivs.1001016
Copyright License: © 2019 Atanásio-Nhacumbe A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Prevalence; Seasonal variations; Gastrointestinal nematodes; Goats; Smallholder farms
Prevalence and seasonal variations of eggs of gastrointestinal nematode parasites of goats from smallholder farms in Mozambique
Alsácia Atanásio-Nhacumbe1* and Carlos Francisco Sitoe2
1National Centre for Biotechnology and Biosciences (CNBB), Ministry of Science and Technology, Higher Education and Vocational Training (MCTESTP), Av. Patrice Lumumba, 770, Maputo, Mozambique
2Animal Sciences Directorate (DCA), Agricultural Research Institute of Mozambique (IIAM), Av. de Moçambique, 1922, Maputo, Mozambique
*Address for Correspondence: Alsácia Atanásio-Nhacumbe, National Centre for Biotechnology and Biosciences (CNBB), Ministry of Science and Technology, Higher Education and Vocational Training (MCTESTP), Av. Patrice Lumumba, 770, Maputo, Mozambique, Tel: +258 21 498813; +258 843221004; Email: [email protected]; [email protected]
A survey was carried out to determine the prevalence and seasonal variations of eggs of gastrointestinal nematodes in goats in four provinces of Mozambique, from November 2016 to October 2017 in Tete and Cabo Delgado, and from November 2016 to October 2018 in Maputo and Gaza. In each province, flocks were selected from both lowlands, located within the valleys of the rivers, and uplands which are located outside the valleys. Faecal samples were collected at monthly intervals to monitor faecal egg counts fluctuations. The modified McMaster technique was used for quantitative analysis of nematode eggs and for detecting cestode eggs in faecal samples. The sedimentation technique for detecting trematode eggs in faecal samples was used. A total of 2 703 samples were examined for nematode eggs and 2 587 for trematode eggs. Faecal examination indicated that between 18% and 100% of goats sampled were infected with gastro-intestinal nematodes. The prevalence varied according to the season of the year and the ecological conditions. The highest prevalence and worm egg counts were recorded at about the peak of the rainy season. Strongyloides papillosus, Calicophoron spp., Fasciola spp. and Schistosoma mattheei eggs were also found. Moniezia expansa and Moniezia benedeni eggs were found in all the four study areas but its prevalence was low and irregular. Based on the results of this study, which showed a clear seasonal pattern, strategic anthelmintic medications to effectively control helminth infections in goats in the different ecological zones of Mozambique are suggested.
Internal helminth parasites are responsible for a significant amount of pathology in farmed animals, causing decreases in small-stock productivity [1].
Parasites are responsible for a wide range of effects, including reduced appetite and lowered feed intake, loss of blood and plasma proteins into the gastrointestinal tract, disturbances in protein metabolism, depressed levels of minerals and reduced activity of some intestinal enzymes. Poor growth and outright loss of weight is one of the most common outcomes of internal parasitism, and if infection is severe enough or sufficiently prolonged, death ensues [2].
Gastrointestinal parasitic infections are world-wide problem for both small and large-scale farmers, but their impact is greater in Sub-Saharan Africa due to the availability of a wide range of agroecological factors suitable for diversified hosts and parasite species [3].
The absence of strategic control programs generally results in an indiscriminate use of anthelmintics, which may have little or no impact on parasite populations. Therefore, the design of strategic parasite control programs requires knowledge of the dynamics of egg shedding from the host [4], and thus the epidemiology of the helminths.
Knowledge concerning epidemiological infection patterns caused by gastrointestinal parasites is essential in the development of appropriate control strategies and this has a potential to reduce production losses [5]. Under conditions of high moisture and warm temperatures, e.g. humid tropical areas, the development and survival of larvae may be high, but, in regions characterized by hot and dry intervals e.g. tropical areas, the development and survival of larvae is reduced. Modern control strategies exploit this phenomenon by timing the administration of anthelmintics so that contamination of the pasture by eggs is reduced, to coincide with adverse climatic conditions which lower the chances of development and survival of larvae [6].
Therefore, the objective of this investigation was to study the epidemiology of helminthes of goats in four ecological zones of Mozambique in order to design measures for its strategic control.
Study areas
The study was conducted in four of the ten provinces of Mozambique, namely Cabo Delgado, Tete, Maputo and Gaza, where 24,1%, 15%, 10,1% and 5,8% of the small ruminant population, respectively, occur, from November 2016 to October 2017 in Tete and Cabo Delgado, and from November 2016 to October 2018 in Maputo and Gaza. The study areas were chosen because of distinct ecological conditions. In each province both lowland (areas within river valleys, referred as Zone 1) and upland zones (areas outside of the river valleys, referred as Zone 2), were included in the study.
In Maputo province three flocks were selected at Boane (lowland) and two at Mahubo (upland); in Gaza province three flocks were selected from Magula (lowland), one from Chicumbane and one from Limpopo Barra (uplands); in Tete province two flocks were selected from Chingodzi (lowland) and three from Kampango (uplands) and in Cabo Delgado three flocks were selected from Mieze (lowland), one from Nanhimbe and one from Maringanha, both uplands. Lowlands are found in the valleys of Umbeluzi river (Maputo), Limpopo river (Gaza), Zambeze river (Tete) and Mieze river (Cabo Delgado), respectively. It follows that the upland zones are drier than the lowlands.
Study animals
A total of 440 goats of both sexes and various ages were selected from the family sector for the study in the four provinces; 74 in Maputo, 134 in Gaza, 100 in Tete and 132 in Cabo Delgado, respectively. A total of twenty flocks, consisting of five flocks in each province, each with 8 to 34 goats were involved in the survey. The animals were mainly a local breed, the Landim goats, and animals were identified by ear tags with code numbers. In 50% of animals the monthly faecal nematode egg count fluctuations were monitored in both lowlands (zone 1) and uplands (zone 2). All farms were visited once a month and faecal samples were collected from the rectum of each animal. The animals surveyed in this study had not been dewormed during the study.
Faecal examination
The Reinecke’s [7] modification of the McMaster technique was used for quantitative analysis of nematode eggs in faecal samples. Two g of faeces and 58 ml sugar solution were mixed in a blender for 10-20 seconds, and 4 to 6 drops of amyl alcohol added to the suspension. A wide-mouthed pipette was used to transfer the suspension to two chambers of the McMaster slide and the slide allowed to stand for two minutes to allow the eggs to float to the top. The McMaster slide was examined under a standard microscope at 10x magnification. The final number of eggs per gram of faeces (epg) was calculated using the formula epg = Te / Tc x 200, where Te is the number of eggs and Tc the number of chambers examined [8].
The sedimentation technique for detecting trematode eggs in faecal samples was used. A 2 g sample of faeces was mixed with 40-50 ml of water. The faecal suspension was passed through a sieve with 0,150 mm apertures and the filtered material put into a 100ml sedimentation tube and allowed to stand for 10 minutes. The supernatant was carefully decanted and the sediment resuspended in water. This was repeated until the supernatant was clear. The supernatant was then finally decanted and the sediment stained with one drop of 1% methylene blue. It was examined on a microscope slide under standard microscope at 10x magnification [9,10]. A total of 2 703 samples were examined for nematode eggs and 2 587 for trematode eggs.
A guideline provided by Hansen & Perry [10] was used for interpretation of faecal egg counts, thus, the degree of infection (eggs per gram of faeces) for mixed infections was classified as light when the egg counts were less than 500, moderate between 500 and 1 000, and heavy when the egg counts were 1 000 or more.
Statistical analysis
Student’s t-test and analysis of variance (ANOVA) were used to evaluate the results using the Statistical Package for Social Science (SPSS; version 21, IBM software, USA) and the significance level for both statistical methods was 5%.
Between 18% and 100% of the goats were infected with gastrointestinal nematodes, depending on the season of the year and on the ecological conditions (Figures 1-4).
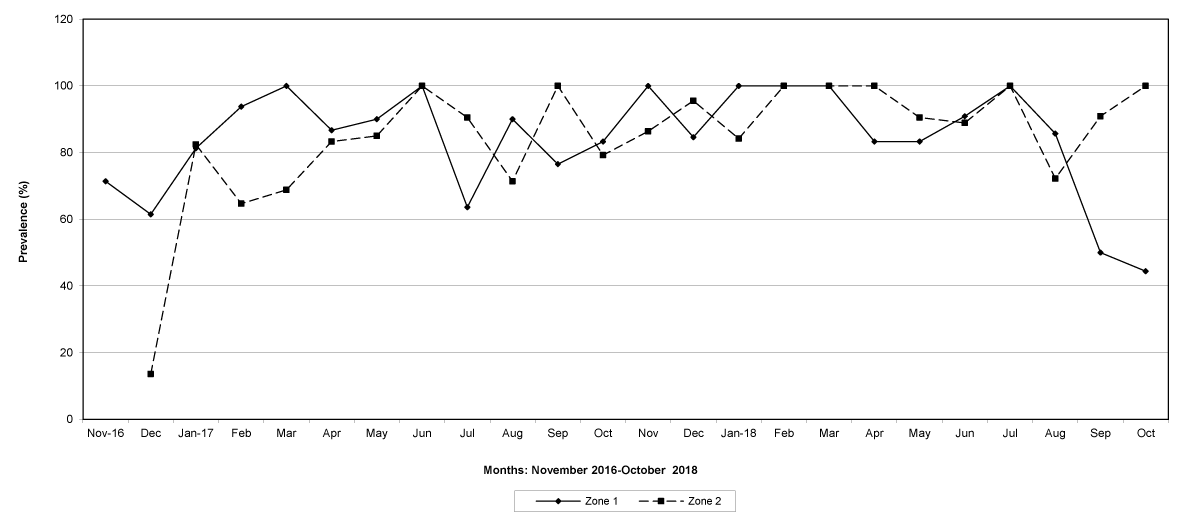
Figure 1: The prevalence of trichostrongylid infections in zones 1 and 2, Maputo Province. No data were available for zone 2 in November 2016.
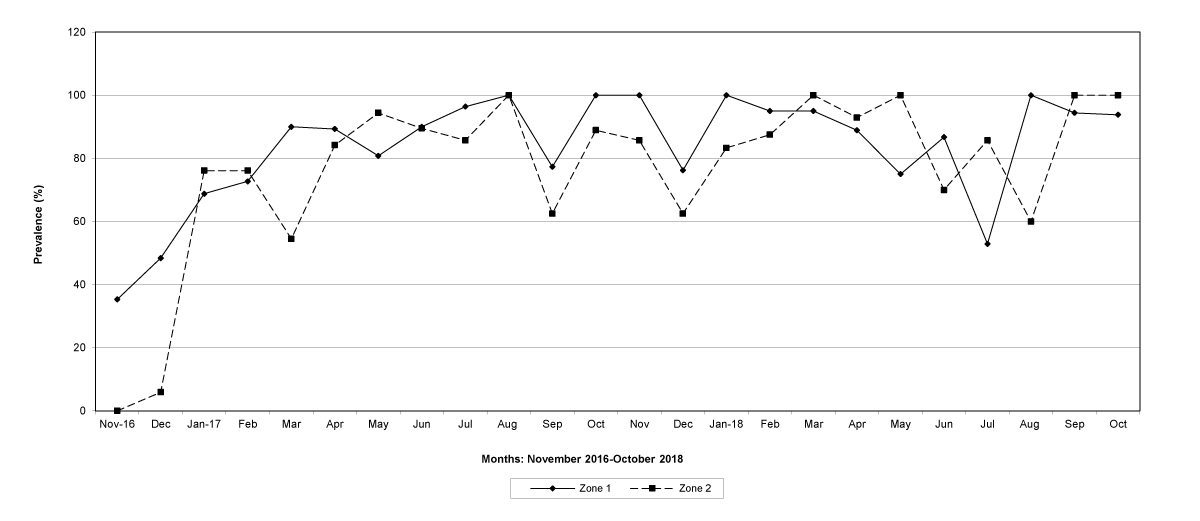
Figure 2: The prevalence of trichostrongylid infections in zones 1 and 2, Gaza Province.
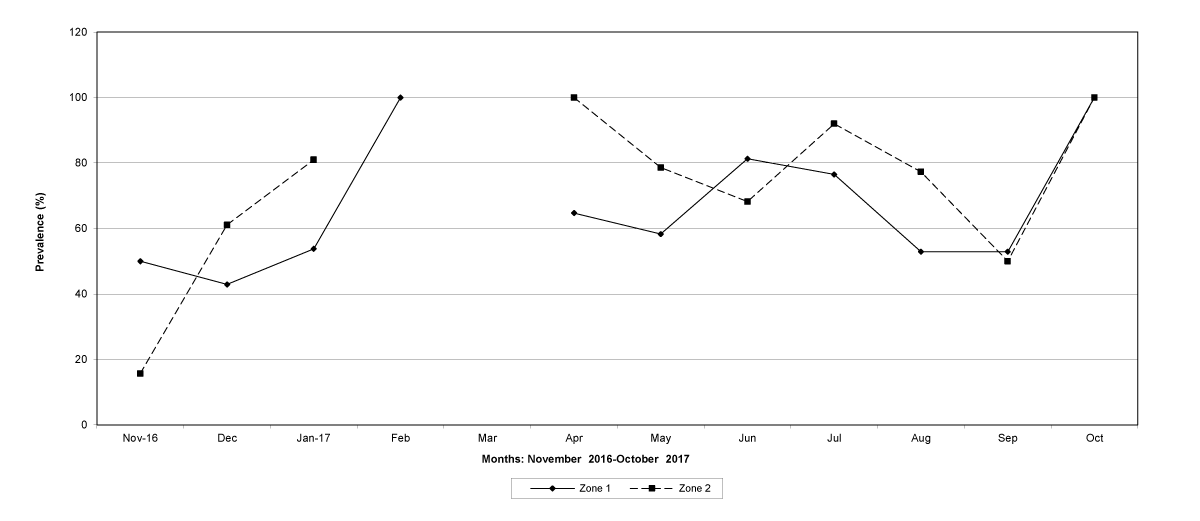
Figure 3: The prevalence of trichostrongylid infections in zones 1 and 2, Tete Province. No data were available for zone 1 (March 2017) and for zone 2 (February and March 2017).
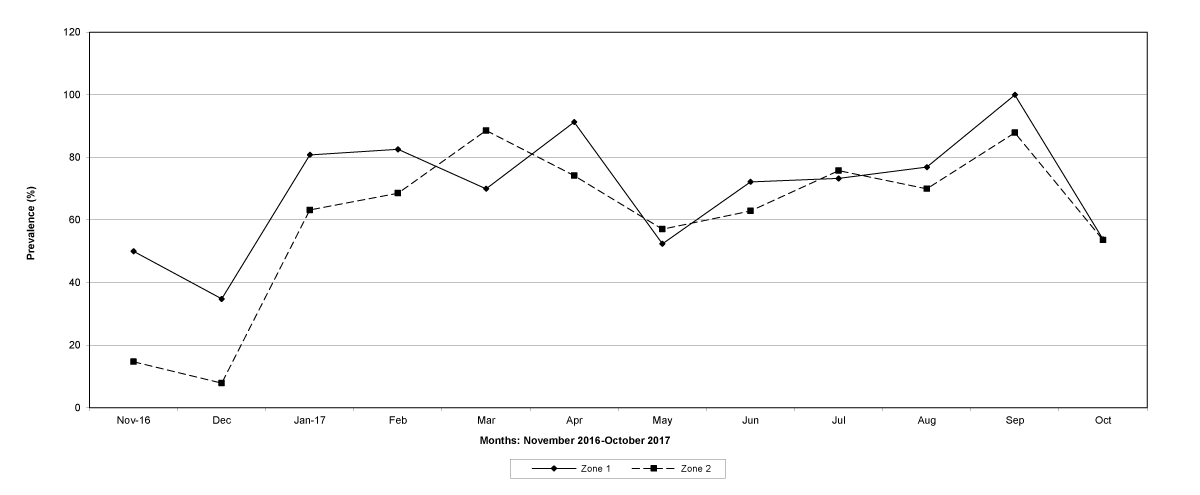
Figure 4: The prevalence of trichostrongylid infections in zones 1 and 2, Cabo Delgado Province.
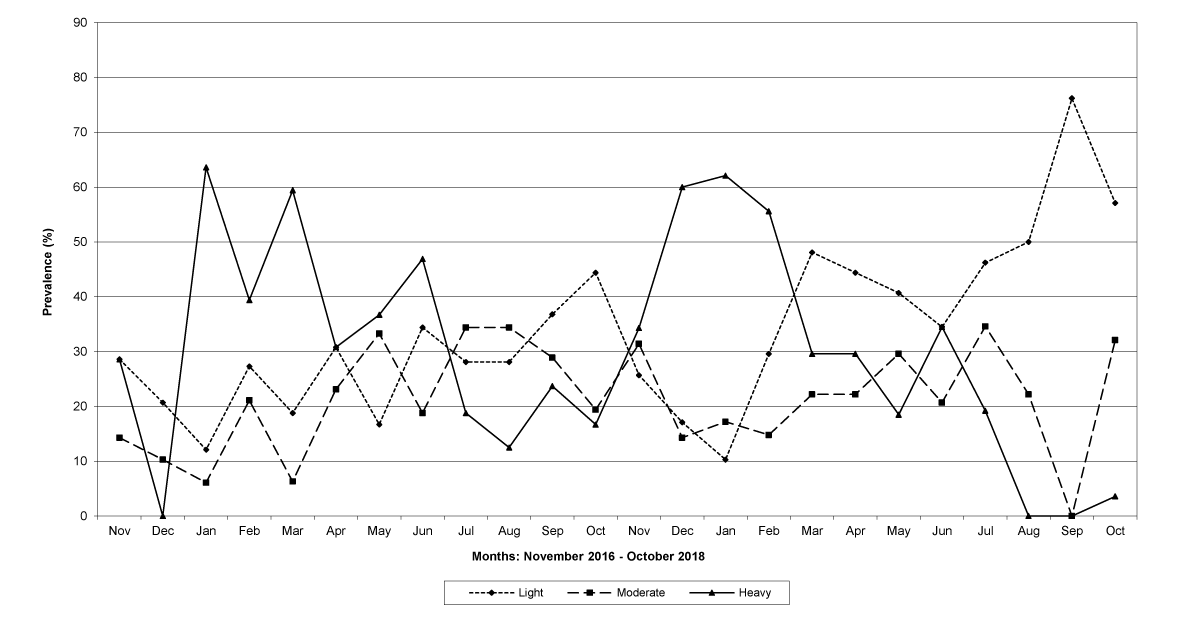
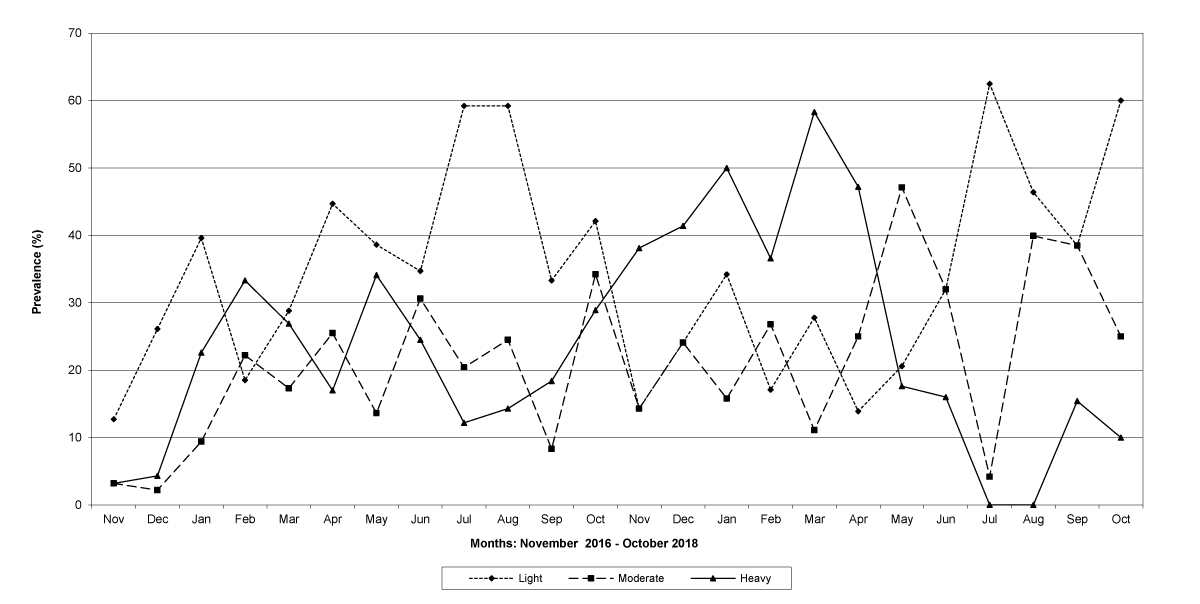
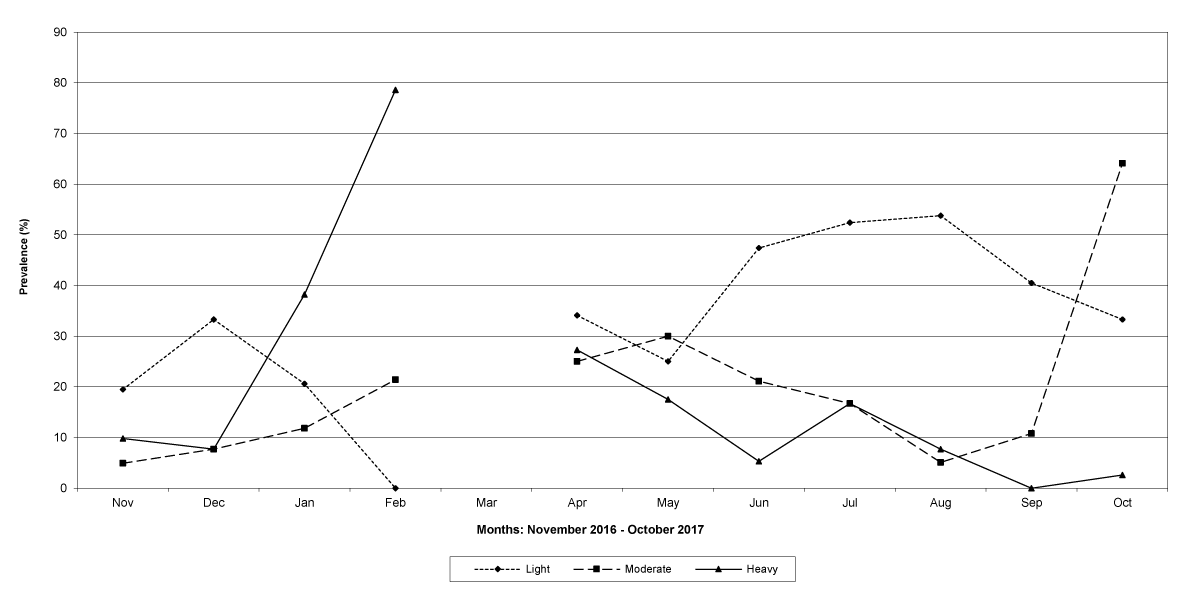
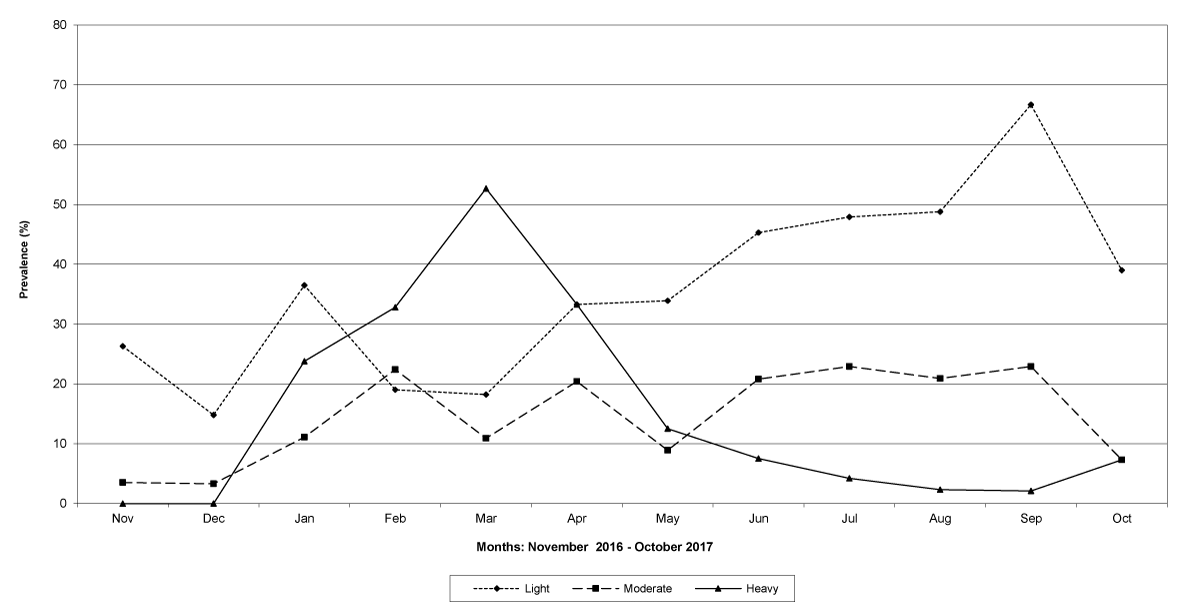
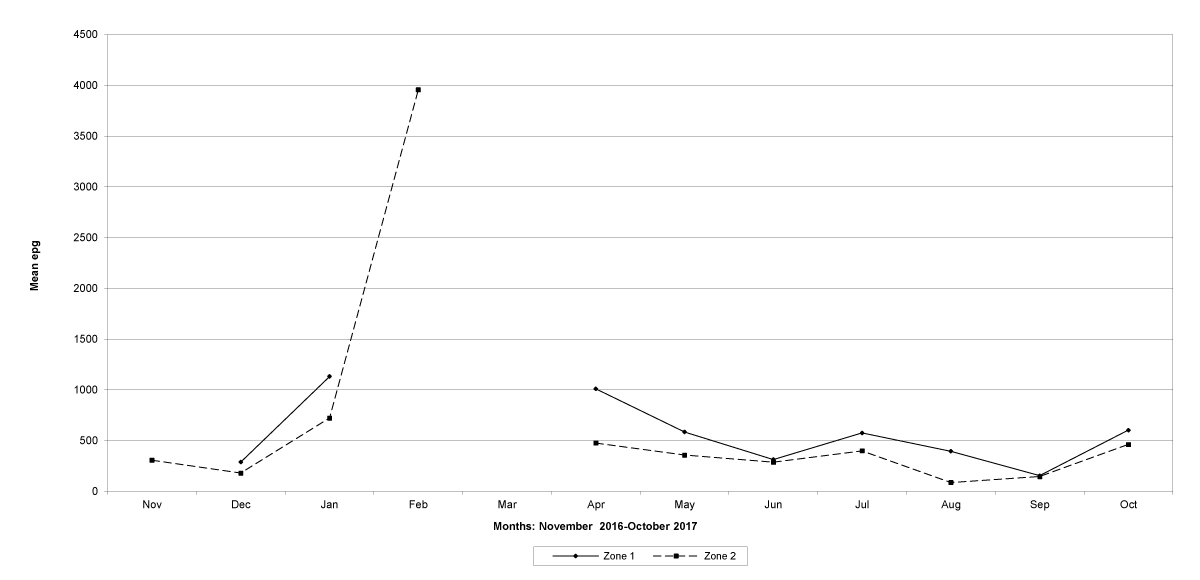
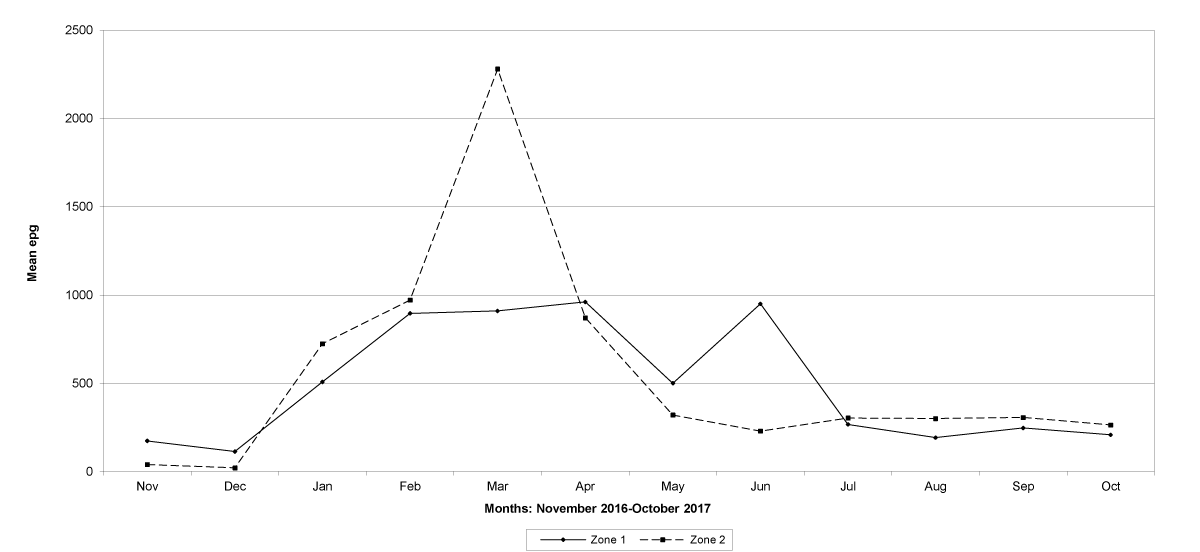
The prevalence of infected goats was high during the wet season in all the four provinces, reaching peaks in January 2017 and January 2018 in Maputo; in February 2017 and March 2018 in Gaza Province; in February 2017 in Tete, and in March 2017 in Cabo Delgado (Figures 5-8).
Figure 5: Prevalence of light, moderate and heavy infections by Trichostrongylids in Maputo Province.
Figure 6: Prevalence of light, moderate and heavy infections by Trichostrongylids in Gaza Province.
Figure 7: Prevalence of light, moderate and heavy infections by Trichostrongylids in Tete Province. No data were available for March 2017.
Figure 8: Prevalence of light, moderate and heavy infections by Trichostrongylids in Cabo Delgado Province.
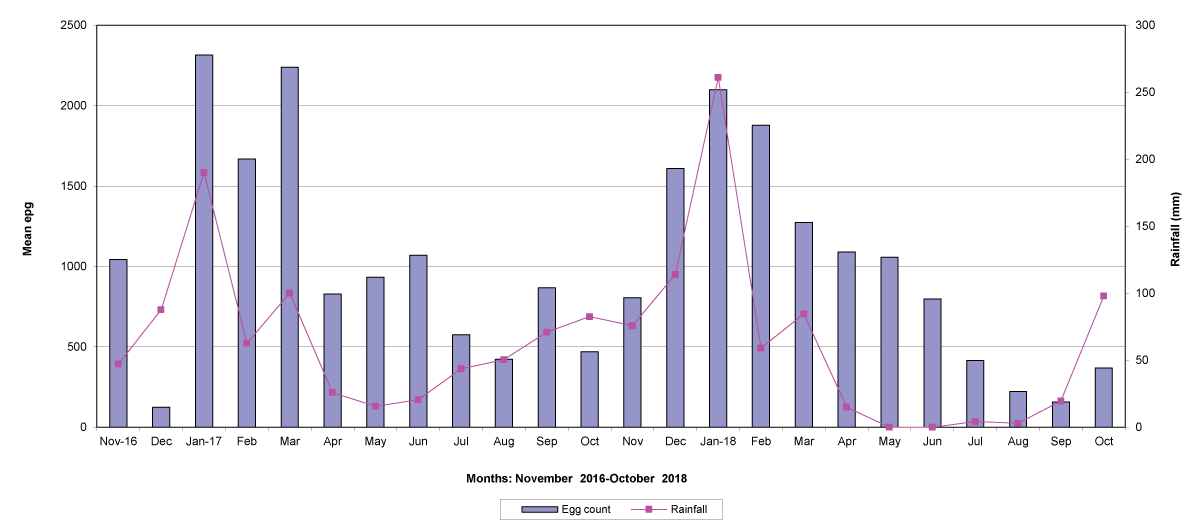
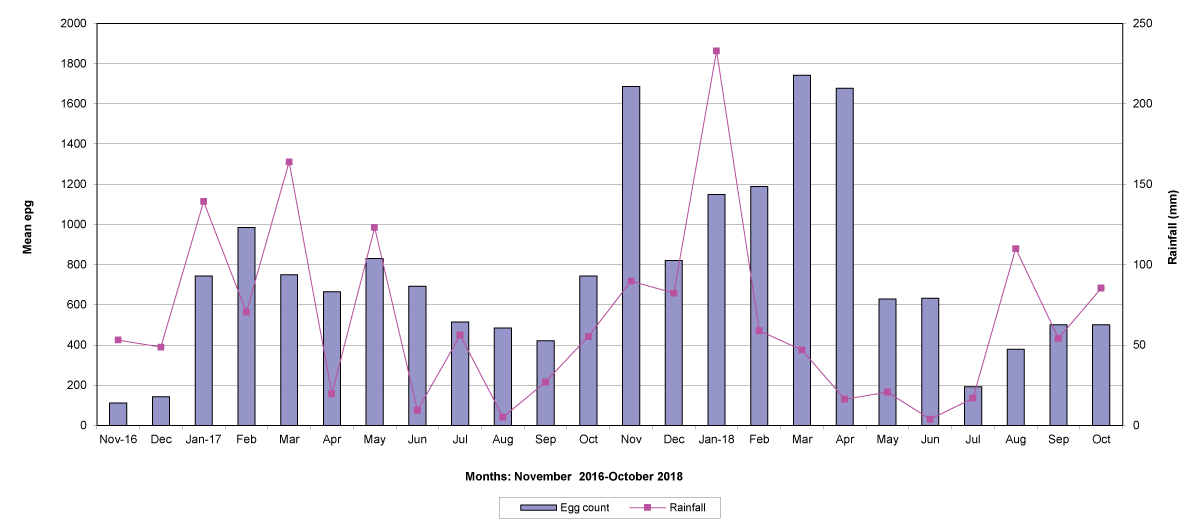
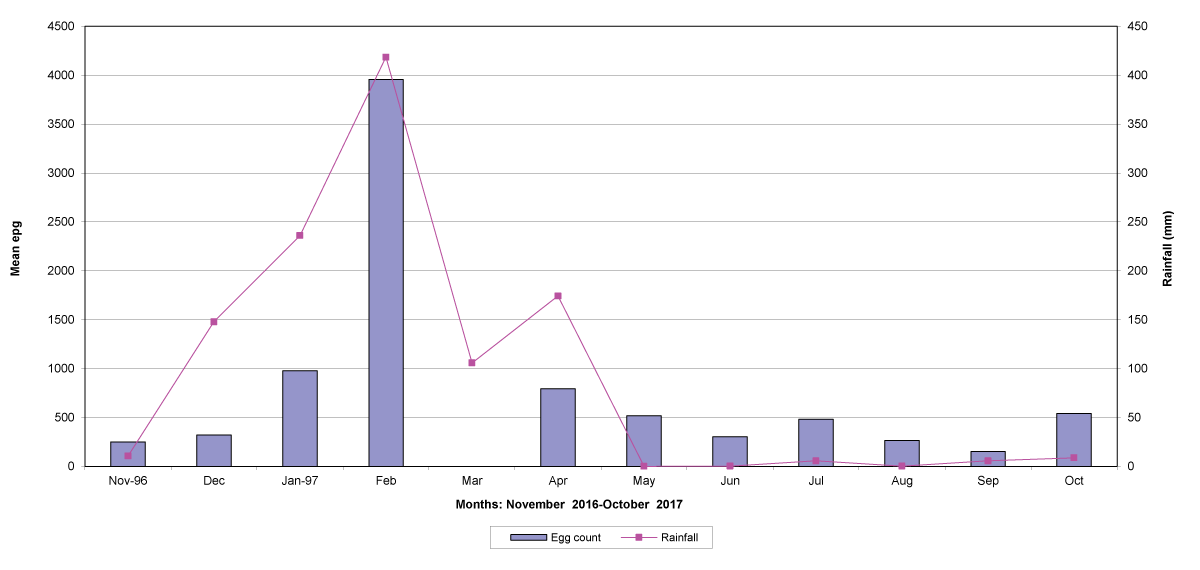
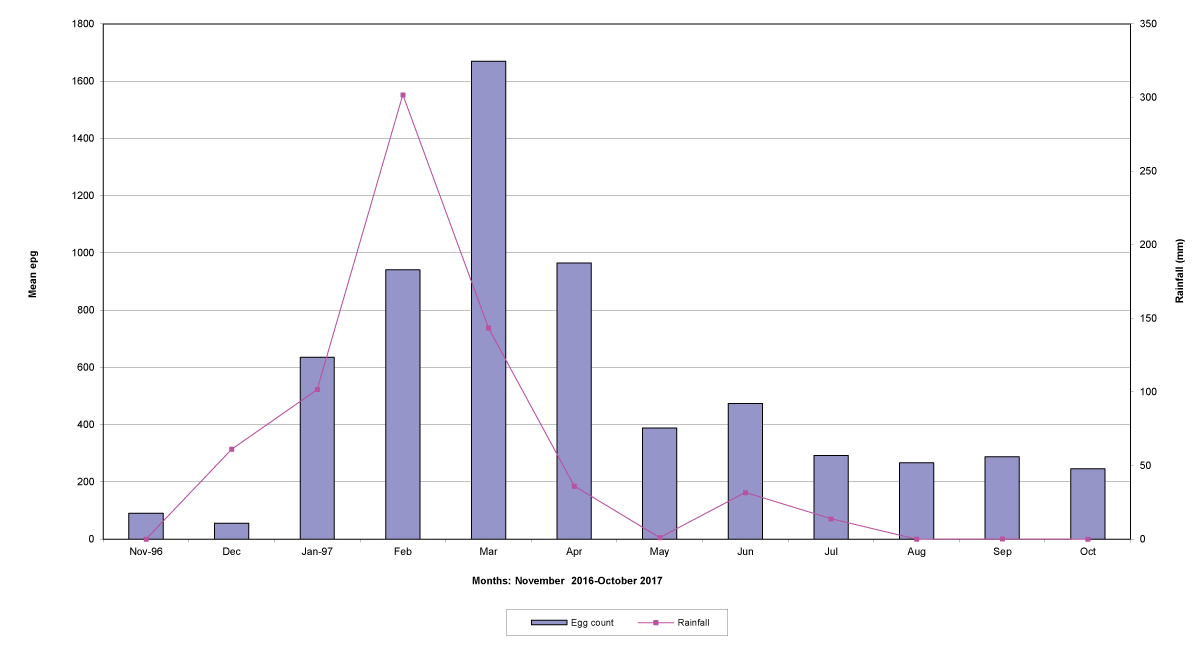
The means of the worm egg counts decreased during November and December 2016, but increased during the rainy season to reach peak values in January 2017, March 2017 and January 2018 in Maputo; during February 2017, November 2017 and March-April 2018 in Gaza; in February 2017 in Tete, and in March 2017 in Cabo Delgado. The seasonal fluctuation of the mean egg counts are illustrated in Figures 9-12. From these figures it is evident that the egg counts in all the four Provinces were lowest during the dry season and highest during the wet season. When the rainfall is higher, the availability of infective larvae on pasture is also higher; therefore, the peaks of the mean faecal egg counts are also highest. The differences between the provinces and between the zones and seasons, however, were not statistically significant (p>0.05).
Figure 9: Mean trichostrongylid egg counts and rainfall in Maputo Province.
Figure 10: Mean trichostrongylid egg counts and rainfall in Gaza Province.
Figure 11: Mean trichostrongylid egg counts and rainfall in Tete Province. No data were available for March 2017.
Figure 12: Mean trichostrongylid egg counts and rainfall in Cabo Delgado Province.
The lowest egg counts occurred during November 2016 in Gaza, December 2016 in Maputo and Cabo Delgado, and during September 2017 in Tete.
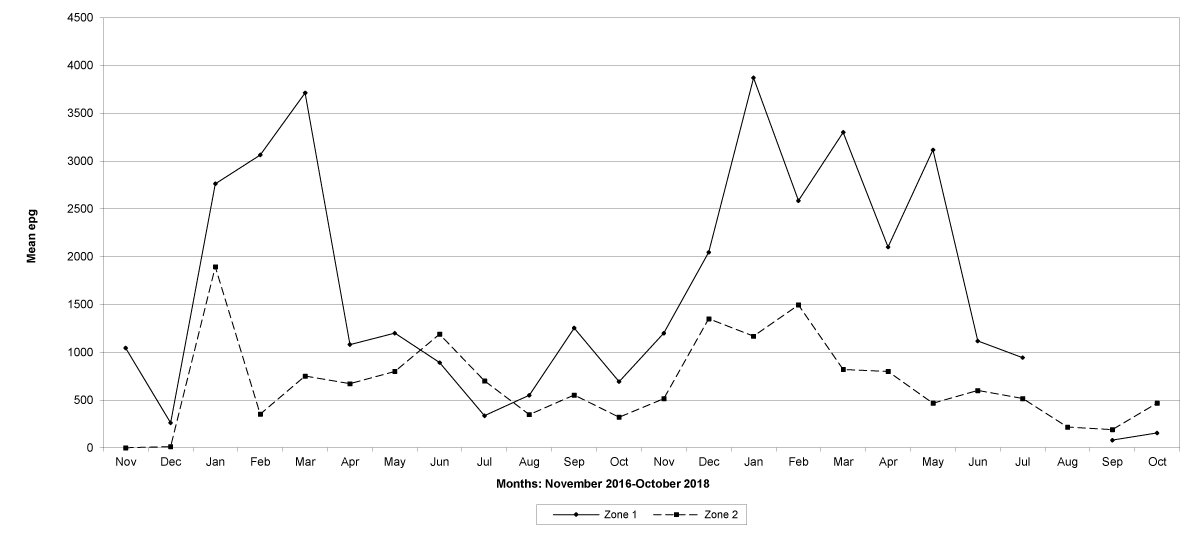
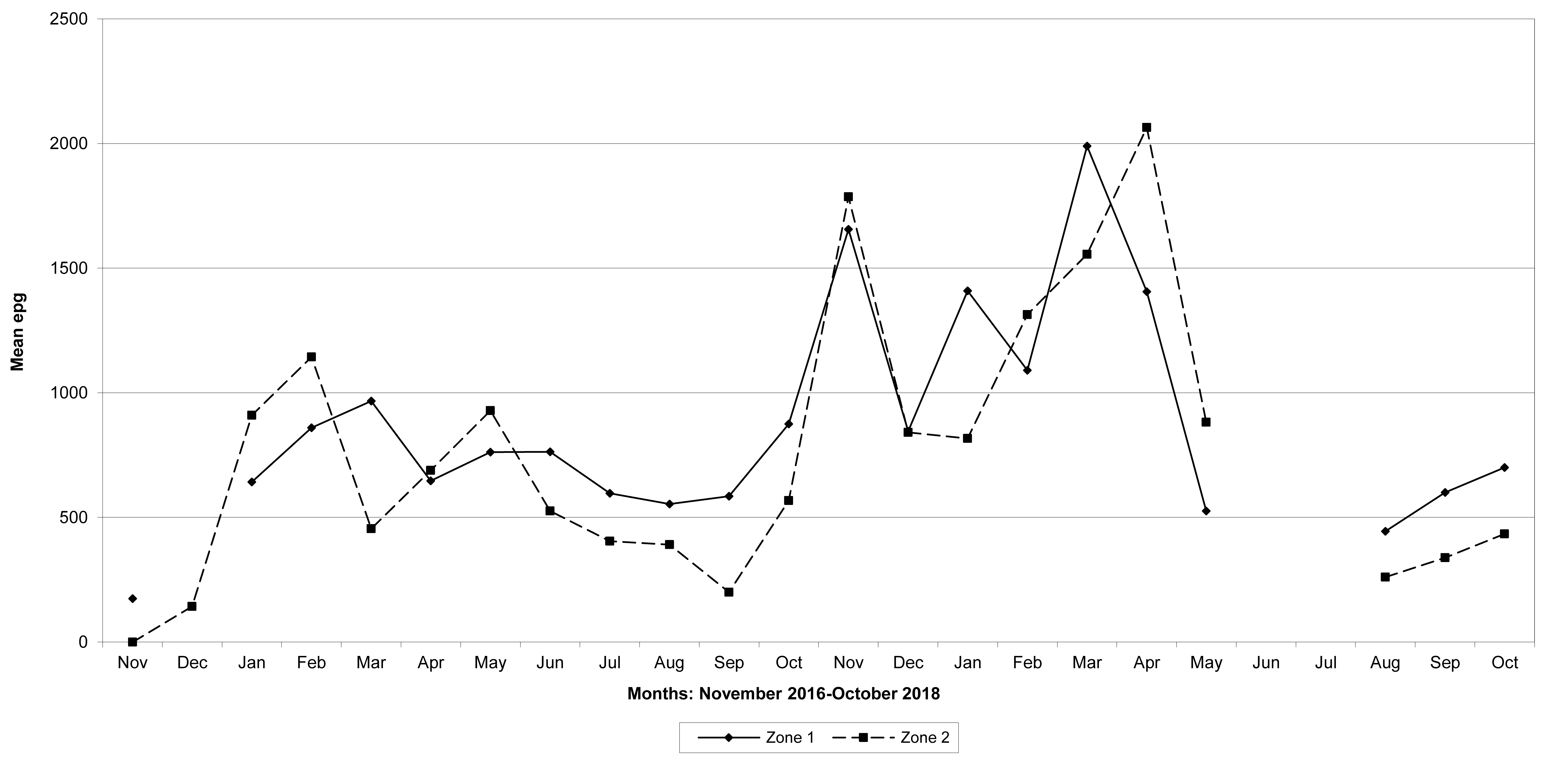
Goats in the lowland farms yielded more nematode eggs than those in the uplands (Figures 13-16).
Figure 13: Mean trichostrongylid egg counts in zones 1 and 2, Maputo Province. No data were available for zone 1 in August 2018.
Figure 14: Mean trichostrongylid egg counts in zones 1 and 2, Gaza Province. No data were available for zone 1 in December 2016, June and July 2018, and for zone 2 in June and July 2018.
Figure 15: Mean trichostrongylid egg counts in zones 1 and 2, Tete Province. No data were available for zone 1 in November 2016, February 2017 and March 2017, and for zone 2 in March, 2017.
Figure 16: Mean trichostrongylid egg counts in zones 1 and 2 in Cabo Delgado Province
A high overall prevalence of S. papillosus was seen in Tete province, being 78,6% in February 2017, 63,6% in April 2017, 55,0% in May 2017, 50,0% in June 2017 and 57,1% in July 2017, respectively. Its prevalence was relatively low in the other provinces, with a maximum of 37,5% in March 2017 in Maputo, 24,1% in November 2017 in Gaza, and 39,3% in May 2017 in Cabo Delgado.The peaks of the mean egg counts occurred in March 2017 (206 epg) in Maputo, in March 2017 (158 epg) in Gaza and in April 2017 (337 epg) in Cabo Delgado. The highest mean egg count of 1 157 epg was observed in February 2017 in Tete province.
The trematode eggs found were those of Calicophoron spp., Fasciola spp. and Schistosoma mattheei. The prevalence of Calicophoron spp. eggs was, in general, low but increased during the wet season reaching 90,0% in January 2017 in Maputo, 59,0% in January 2017 and 77,8% in October 2017 in Gaza. Very low prevalence occurred in Tete with a peak of 26,5% in January 2017, and 10,0% in November 2016 in Cabo Delgado. Calicophoron eggs occurred throughout the year, although with fluctuations, in Maputo and Gaza, while in Tete and Cabo Delgado these eggs were found only during the wet season.
Fasciola eggs were detected in Maputo only in April 2017 (2,7%) and in January 2018 (3,7%). In Gaza, a prevalence of 20,5% in January 2017, 5,7% in February 2017, 2,4% in April 2017 and 8,8% in December 2017 was observed. In Tete, eggs were seen only in January 2017 (26,5%) and in Cabo Delgado these eggs were detected only in December 2016 (7,7%) and in January 2017 (5,2%).
Only three positive cases of S. mattheei were found, two in January 2017 in Gaza and one in February 2017 in Maputo Province.
M. expansa and M. benedeni eggs were found in all the four provinces but their prevalence was very low, and their presence irregular. The highest prevalence of M. expansa eggs occurred during December 2016, it being 20,7% in Maputo and 10,9% in Gaza. In Tete and Cabo Delgado they occurred during July 2017 (4,8%) and February 2017 (8,6%), respectively. The prevalence of M. benedeni eggs was lower than that of M. expansa in the provinces of Tete and Cabo Delgado, with peak numbers (2,6%) occurring during August 2017 and February 2017 (3,4%) respectively. However, M. benedeni eggs were often encountered in Maputo and Gaza, especially during November 2016 (28,6%) in Maputo and September 2017 (10,5%) in Gaza.
This study on helminth infection pattern in goats has been carried out in traditional husbandry system and covering different ecological zones of Mozambique. Specht [11] studied the helminth infection pattern of sheep and goats in southern Mozambique. However, the study was carried out at a small ruminant research centre in Maputo where animals were kept under semi-intensive conditions. Furthermore, the study involved only few animals; thus, her findings give an indication of the occurrence of these infections but cannot be directly compared with the results of a study of this extent.
In a study of patterns of disease in smallholder goat farms in Mozambique, Costa [12] found that of 93 goats examined, 26,0% had gastroenteritis due to helminths. Clinical disease and deaths due to helminths were also seen. Furthermore, this author reported that of the most common diseases clinically diagnosed, enteric diseases, mostly in connection with gastrointestinal parasites accounted for 31,8% of the total causes of morbidity. Some animals presented signs of helminthoses, and some died during the course of this study, but no confirmation was made at laboratorial level. The results observed in our study also revealed that helminthic infections are of importance in the health of goats in the family sector in Mozambique when their prevalence and the eggs yielded by the animals are taken into account.
The prevalence of parasites varies according to ecological conditions. High prevalence was observed in zones 1 (lowland) and 2 (upland), however, the prevalence of egg counts was relatively higher in more humid areas (lowlands) than in uplands in the Provinces of Maputo, Gaza and Cabo Delgado. In Tete, a semi-arid area where little variations in the humidity occurred between the two zones, no distinct differences in the prevalence were evident between the two zones. In a humid tropical area of India for example, almost 100% of grazing sheep and goats harbour light to medium levels of parasites [13].
From the results it is evident that high rainfall is associated with high egg counts. This may be expected, as in the tropics the limiting ecological factor influencing the severity of parasitism is rainfall, as temperatures almost always favour hatching and development of the free-living stages [14,15]. Our observations agree with those of Fagbemi & Dipeolu [16]; Specht [11]; Vercruysse [17]; Asanji [18] and Suarez & Buseti [19]. Macpherson [20] also states that environmental conditions, especially relative humidity and temperature, profoundly influence parasitic diseases in more arid areas. Our results are in agreement with those of Nwosu et al. [21], who reported that the prevalence and counts of trichostrongylid nematode eggs showed a definite seasonal sequence that corresponded with the rainfall pattern, and counts of trichostrongylid egg type increased with the rains and reached peak levels at about the peak of the rainy season in the semi-arid zone of north-eastern Nigeria.
Our observations also are in agreement with the findings of Connor, Munyuku, Mackayo & Halliwell [22], who in an investigation on the epidemiology and control of helminthoses in southern Tanzania found that the mean egg counts remained below 500 epg until December when the rainy season began, and the highest group mean egg count was recorded in February. In Cabo Delgado Province which is closer to the southern region of Tanzania, the highest egg counts were observed in March 2017 and then a sharp decrease occurred following the sharp decline in rainfall. Similar results were observed also in Nigeria by Nwosu, Ogunrinade & Fagbemi (1996). In a study also conducted in Tanzania, Shija et al., [23] reported that the trichostrongylid were the most prevalent parasites and the prevalence was high in the rainy season, which is in agreement with our study where high infections were observed during the wet season.
Goossens, Osaer, Kora, Chandler, Petrie, Thevasagayan, Woolhouse & Anderson [24] also found that faecal egg counts of trichostrongylid nematodes were highest during the rainy season in goats in The Gambia. This country is situated in a semi-arid zone in West Africa with distinct dry (December to June) and wet (July to November) seasons. These climatic conditions are comparable with those of Tete Province which is located in a semi-arid zone of Mozambique, and the dry season is distinct (April to November). In this Province the faecal egg counts of trichostrongylid nematodes were also highest during the rainy season (February 2017) but declined in March as the rains also declined.
In Maputo and Gaza Provinces, the mean egg counts were higher from January to April when the rainfall was also higher. In this Province the wet season is more extended than in Tete and Cabo Delgado, thus, the egg counts remained higher for longer. This agrees with the results of Fivaz, Horak & Williams [25] who observed that in the Eastern Cape, egg counts of Angora goats were higher during the rainy season, from March to July 1988.
Both the prevalence and the mean egg counts differed among the different ecological zones, and within the different ecological conditions they varied between lowland and upland zones. According to Rossanigo & Gruner [26], temperature and moisture are important factors influencing the development of the free-living stages of gastrointestinal nematodes of ruminants. Horchner [27] also refers that the intensity of contamination and infection is not only dependent on the season but also on the local ecological situation.
In the four provinces the mean epg varied greatly between the low and uplands, showing the importance of microclimate in the infection rate. According to Thrusfield [28], the degree of infection by helminths can be affected by the microclimate. In lowlands, the high humidity favors the development of larvae compared with that in the uplands, and this is evidenced by higher egg counts.
The prevalence of S. papillosus varied in the four Provinces but it was relatively higher during the rainy season as that of egg counts. Fivaz et al., [25], Agyei [29] and Nwosu et al., [16] also found more eggs during the rains. However, the prevalence did not show a clear seasonal pattern during this study.
The occurrence of both M. expansa and M. benedeni was irregular. It is well known that the availability of the intermediate host, oribatid mites, is essential for the occurrence of these cestodes. The age of the animals also influence the occurrence of these cestodes. Fivaz et al., [25] in South Africa and Agyei [29] in Ghana also observed that the presence of these cestodes did not show a definite seasonal pattern, which confirm our results.
Calicophoron spp. eggs were present throughout the year but increased in numbers during the wet season in Maputo and Gaza, while in Tete and Cabo Delgado it occurred only during the wet season. This is in accordance with Nwosu et al., [16] who encountered more eggs of this parasite during the rainy than in the dry season.
Only three positive cases of S. mattheei were found during this study. This finding makes it impossible to draw conclusions either about the occurrence of this trematode or its importance in the health of goats in this country. Schistosoma spp. is dependent upon water as medium for infection of both the intermediate and final host [30]. One of the probable reasons for the occurrence of such a few cases could be the location of the flocks surveyed. In one of the flocks located near the Limpopo River in Gaza, for example, clinical cases of schistosomoses were registered in sheep and goats during January 2017. From the affected animals one sheep died, and several parasites were found in the mesenteric veins. The other affected animals recovered after had been treated with praziquantel for humans by the local Veterinarian.
Fasciola eggs were found in the four study locations, although in very low numbers. This agree with Atanásio [31] that reported the occurrence of these eggs for the first time in goats from Mozambique, since Cruz e Silva [32] and Specht [11] do not refer the occurrence of this parasite in goats in this country. In Zimbabwe, a neighbouring country of Mozambique, Fasciola spp in goats from low-input low-output farming systems was also reported by Zvinorova et al. [5].
The data provided in this study, which show a clear seasonal pattern of helminth egg counts is of epidemiological significance. They are the base for the design of control measures exploiting these variations in order to allow a control of helminths, and therefore a sustainable use of anthelmintics.
The trichostrongylid egg output was higher during the peak of the rains but peaks occurred during different months, according to the ecological conditions prevailing in each study area.
Therefore, based on the results of this survey, which showed a clear seasonal pattern, anthelmintic medications to effectively control helminth infections in goats in the different ecological zones of Mozambique suggested are the following:
1) In Maputo, medication should be given during November, January, March and June;
2) In Gaza, during November, January, March and May;
3) In Tete, during January and April;
4) And in Cabo Delgado, during January, March and June.
However, since systematic deworming can lead to anthelmintic resistance and does not seem viable for implementation at the traditional husbandry systems, where generally the worm burdens are negligible, the treatment of clinical cases of helminthoses individually is recommended, and in young animals and females which are more susceptible to helminthoses. Furthermore, the control measures suggested in this study could be applied in the private and commercial sectors where helminths jeopardize the animals, and the farmers can afford buying anthelmintics for drenching the animals on a regular basis.
This study was conducted with the support of the Swedish International Development and Cooperation Agency (SIDA). The technical assistance by the staff of the Parasitology Section at Animal Sciences Directorate/IIAM, and by the technicians from the Provincial Veterinary Laboratories in Gaza, Tete and Cabo Delgado is also acknowledged.
- Fraser A, Craig PS. Detection of gastrointestinal helminth infections using coproantigen and molecular diagnostic approaches. J Helminthol. 1997; 71: 103-107. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9192710
- Carmichael IH. Internal and external parasites as constraints to productivity of small ruminants in the humid tropics. In: (Eds.) Manika Wodzicka-Tomaskewska, Susan Gardiner, A. Djajanegara, I.M. Mastika & T.M. Wiradarya. Sebelas Maret. Small ruminant production in the humid tropics. University Press. 1993; 285-335.
- Owhoeli O, Elele K, Gboeloh LB. Prevalence of gastrointestinal helminths in exotic and indigenous goats slaughtered in selected abattoirs in Port Harcourt, South-South, Nigeria. Chinese Journal of Biology. 2014; 2014: 435913.
- Thamsborg SM, Roepstorff A, Larsen P. Integrated and biological control of parasites in organic and conventional production systems. Vet Parasitol. 1999; 84: 169-186. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10456414
- Zvinorova PI, Halimani TE, Muchadeyi FC, Matika O, Riggio V, et al. Prevalence and risk factors of gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin Res. 2016; 143: 75-83. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27766016
- Anon. FAO expert consultation on helminth infections of livestock in developing countries. Rome, Italy, 23-27 September 1991. Food and Agriculture Organization of the United Nations.
- Reinecke RK. Helminth research in South Africa. III. The diagnosis of nematode parasites in ruminants for worm survey purposes. J South African Veterinary Medical Association. 1961; 37: 27-31.
- Reinecke RK. Veterinary helminthology. Durban: Butterworths. 1983.
- Dunn AM. Veterinary helminthology. William Heinemann Medical Books. London. Second Edition. 1978; 295-298.
- Hansen J, Perry B. The epidemiology, diagnosis and control of helminth parasites of ruminants. Second Edition. ILRAD, Nairobi, Kenya. 1994.
- Specht EJ. Seasonal incidence of helminths in sheep and goats in south Mozambique. Vet Parasitol. 1982; 11: 317-328. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6892176
- Costa RF. Patterns of disease in small family goat farms in Mozambique. MSc thesis, Faculty of Veterinary Medicine, Swedish University of Agricultural Sciences. 1993.
- Khan MQ, Ghaffar A, Anwar M, Khan MA. Importance of parasites as a constraint on small ruminant production in Pakistan. Report of an international workshop on Sustainable parasite control in small ruminants, held in Bogor, Indonesia. 1997; 22-25.
- Van Aken D, De Bont J, Vercruysse J, Dorny P. Gastro-intestinal nematode infections in a goat breeding farm in North-Western Sri Lanka. Trop Anim Health Prod. 1990; 22: 231-238. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2288007
- Waller PJ. Nematode parasite control of livestock in the tropics/subtropics: the need for novel approaches. Int J Parasitol. 1997; 27: 1193-1201. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9394190
- Nwosu CO, Ogunrinade AF, Fagbemi BO. Prevalence and seasonal changes in the gastro-intestinal helminths of Nigerian goats. J Helminthol. 1996; 70: 329-333. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8960229
- Vercruysse J. A survey of seasonal changes in nematode faecal egg count levels of sheep and goats in Senegal. Vet Parasitol. 1983; 13: 239-244. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6686379
- Asanji MF. Haemonchosis in sheep and goats in Sierra Leone. J Helminthol. 1988; 62: 243-249. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3192917
- Suarez VH, Busetti MR. The epidemiology of helminth infections of growing sheep in Argentina’s Western Pampas. Int J Parasitol. 1995; 25: 489-494. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7635625
- Macpherson CN. Epidemiology and control of parasites in nomadic situations. Vet Parasitol. 1994; 54: 87-102. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7846874
- Nwosu CO, Madu PP, Richards WS. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of north-eastern Nigeria. Vet Parasitol. 2007; 144: 118-124. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17127006
- Connor RJ, Munyuku AP, Mackayo E, Halliwell RW. Helminthosis in goats in southern Tanzania: Investigations on epidemiology and control. Trop Anim Health Prod. 1990; 22: 1-6. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2321258
- Shija DSN, Kusiluka LJM, Chenyambuga SW, Shayo D, et al. Animal health constraints in dairy goats kept under smallholder farming systems in Kongwa and Mvomero Districts, Tanzania. J Vet Med Anim Health. 2014; 6: 268-279.
- Goossens B, Osaer S, Kora S, Chandler KJ, Petrie L, et al. Abattoir survey of sheep and goats in The Gambia. Vet Rec. 1998; 143: 277-281. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9569483
- Fivaz BH, Horak IG, Williams EJ. Helminth and arthropod parasites of Angora goats on irrigated kikuyu grass pastures in the Eastern Cape Province. J S Afr Vet Assoc. 1990; 61: 112-116. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2286996
- Rossanigo CE, Gruner L. Moisture and temperature requirements in faeces for the development of free-living stages of gastrointestinal nematodes of sheep, cattle and deer. J Helminthol. 1995; 69: 357-362. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8583130
- Horchner F. Proposals for epidemiological surveys of helminthosis aimed at the improvement of livestock production in the tropics. Trop Med Parasitol. 1990; 41: 422-424. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2075388
- Thrusfield M. Veterinary epidemiology. Butterworths. Determinants of disease. 1986; 47-75.
- Agyei AD. Epidemiological observations on helminth infections of calves in southern Ghana. Trop Anim Health Prod. 1991; 23: 134-140. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1763433
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Veterinary parasitology. Longman Scientific & Technical. 1987.
- Atanásio A. Helminths, Protozoa, Heartwater, and the Effect of Gastro-intestinal Nematodes on the Productivity of Goats of the Family Sector in Mozambique. PhD thesis in Veterinary Science. Medical University of Southern Africa (MEDUNSA). 2000.
- Cruz e Silva JA. A contribution to the study of animal helminthiasis in southern of Save. Veterinária Moçambicana. 1971; 4: 33-42.