More Information
Submitted: August 20, 2024 | Approved: September 04, 2024 | Published: September 05, 2024
How to cite this article: Haidar CM, Awad A, Diab W, Kanj F, Younes H, Yaacoub A, et al. Comparative Analysis of Water Wells and Tap Water: Case Study from Lebanon, Baalbeck Region. Insights Vet Sci. 2024; 8(1): 018-037. Available from: https://dx.doi.org/10.29328/journal.ivs.1001043.
DOI: 10.29328/journal.ivs.1001043
Copyright License: © 2024 Haidar CM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Boreholes; Potable water; Bacteriological analysis; Water-related diseases
Comparative Analysis of Water Wells and Tap Water: Case Study from Lebanon, Baalbeck Region
Chaden Moussa Haidar1,2*, Ali Awad1,3, Walaa Diab3, Farah Kanj2, Hassan Younes2, Ali Yaacoub4, Marwa Rammal2 and Alaa Hamze1,3
1Islamic University of Lebanon, Khaldeh, Lebanon
2Faculty of Agricultural Engineering and Veterinary Medicine, Lebanese University Dekwaneh, Beirut, Lebanon
3Faculty of Science, Lebanese University, Rafic Hariri University Campus, Hadath, Lebanon
4Industrial Research Institute (IRI), Lebanese University, Rafic Hariri University Campus, Hadath, Lebanon
*Address for Correspondence: Chaden Moussa Haidar, Islamic University of Lebanon, Khaldeh, Faculty of Agricultural Engineering and Veterinary Medicine, Lebanese University Dekwaneh, Beirut, Lebanon, Email: [email protected]
Water deficit is a fundamental factor in public health and economic growth. Water supply and population growth are directly linked to water demand. The physio-chemistry and microbiology analysis of water is utmost significance in dietary requirements. Drinking water has the main concern especially it affects food security. This study includes a number of representative sites where 24 water samples (from wells, reservoirs and tap water) were analysed. These sites are located in the western villages of Baalbeck, the main city of the Bekaa Plain in Lebanon where the analysed water is used mainly for domestic needs and for irrigation. This study investigates the physiochemical and microbiological properties. Among the selected sites, Hawsh Barada site shows a strong contamination by nitrate. At the Nabi Rashaded (tap), Beit Shema and Bednayel (borehole and tap), contamination above the norm by zinc ion was noted. From a microbiological point of view, Hawsh barada, Nabi-Rashadeh, Hawsh-bay, and Beit shema are markedly polluted and do not meet the standard for drinking water. Hence, water quality in Hawsh barada, Nabi-Rashadeh, Hawsh-bay, Beit shema and Bednayel are not suitable for drinking, and this must be informed to decision makers who can act implementing environmental controls for health protection in the studied region.
Water contamination is the major problem in both urban and mostly rural area of Lebanon, notably in the Central Bekaa Region. Most investigated water contains natural and anthropogenic contaminants, particularly inorganic contaminants that arise from rock mineralogy through which water flows and stored to a varying extent [1]. While, the anthropogenic pollution by both microorganism and chemicals. In general, deep groundwater aquifers are less vulnerable to pollution than surface water. Several villages in Central Bekaa are rural ones and they face severe pollution from both commercial and house hold activities leading to severe contamination such as Diarrhea, typhoid, cholera, etc. [2]. This paper aims at assessing the quality of both taps and wellsʹ water in Baalbeck Region which has not been investigated before and adequate supply of safe drinking water is one of the major prerequisites for a healthy life, but waterborne diseases is still one of the challenges in many vulnerable societies, particularly in rural areas[2] . It is also sa significant economic constraint in many subsistence economic.
Drinking water is mainly derived from two basic sources, the surface waters, such as rivers, springs and reservoirs and from groundwater. All water sources contain natural contaminant, particularly inorganic contaminants that arise from the rock lithologies through which water flows and to a varying extent, anthropogenic pollution by both microorganism and chemicals [3].
In general, groundwater is less vulnerable to pollution than surface water. There are a number of possible sources of man-made contaminant, some of which are more important than others, and they fall into the categories of point and diffuse sources. Discharges from industrial premises and sewage treatment work are point sources and as such sources are more readily identifiable and controlled; run off from agricultural land and from rugged surfaces are not so obvious, or easily controlled [4]. Such source can give rise to significant variation in the contaminant load over time. In other words, chemicals, physical, and biological characteristics of water are major indicators in determining either or not water is suitable for domestic, industrial or agricultural use.
Even though, soil and rock stratum are excellent for the mechanism of filtering out particulate matter, such as leave soil and bugs dissolved chemical, yet they can still occur in large enough concentration in ground water to cause problems [5]. Groundwater is often contaminated from the surface materials, and from the organic pollutant, such as petroleum hydrocarbon and detergent which may visibly builds up in an environment as well as the organic chemical, also the breakdowns with time in the environment but metals do not and easily accumulate and concentrated in the living systems [4,5]. There is also the possibility of spills of chemicals from industry and agriculture and slurries from intensive farm units that can contain pathogens. In many regions, severely sited latrines and septic tanks are a significant source of contamination; especially in water wells. In addition, local industries can also give rise to contamination of water sources, particularly when chemicals are handled and disposed without proper care [6]. The run-off or leaching of nutrients into slow flowing surface water sources can result in excessive growth of cyanobacteria or blue-green algae [7]. Many species give rise to nuisance chemicals that cause taste and interfere with drinking water treatment. However, they frequently produce toxins, which are harmful for health; particularly where limited treatment is applied. If treatment is not optimized, unwanted residues of chemicals used in water treatment can also cause contamination and gives chance for sediments to deposit in water pipes [8]. Contaminated drinking water may also arise from materials such as iron, which can corrode to release iron oxide, or from ingress of pollutants into the distribution system. Diffusion through plastic pipes can occur as well, for example, when oil is spilt on the surrounding soil horizons, it gives rise to taste or dour problems [9]. Contamination can also take place in consumers premises from materials used in plumbing, such as lead or copper or from the back water flow into the distribution system as a consequence of improper connections. Such contaminants aspect can be either chemical or microbiological [10].
Drinking water treatment, as applied to public water supplies, consists of a series of barriers in a treatment process that vary according to the requirements of the supply and the nature and vulnerability of such sources [11,12]. Broadly, these comprise system for coagulation and flocculation, filtration and oxidation, where the most common oxidation disinfectant used in chlorine. This provides effective and robust barriers to pathogens and provides an easily measured residual that can act as a marker to show that disinfection has been carried out and acting on preservative in water distribution. The basis on which drinking water safety is judged according to the national standards and norms or international guidelines. In this respect, WHO Guidelines [13] for Drinking Water Quality 2017 is a major advisory; especially they are revised on a regular basis and are supported by a range of detailed documents describing many of the aspects of water safety. The Guidelines are now based on Water Safety Plans that encompass a much more proactive approach to safety from source-to-tap. This work aims to measure the quality of various samples of water from six sites from Baalbek Region. The study was carried out to investigate the quality of potable water and to assess whether it is safe for health or it requires treatment, and this was based on the comparative analysis with WHO norms.
Sites’ description
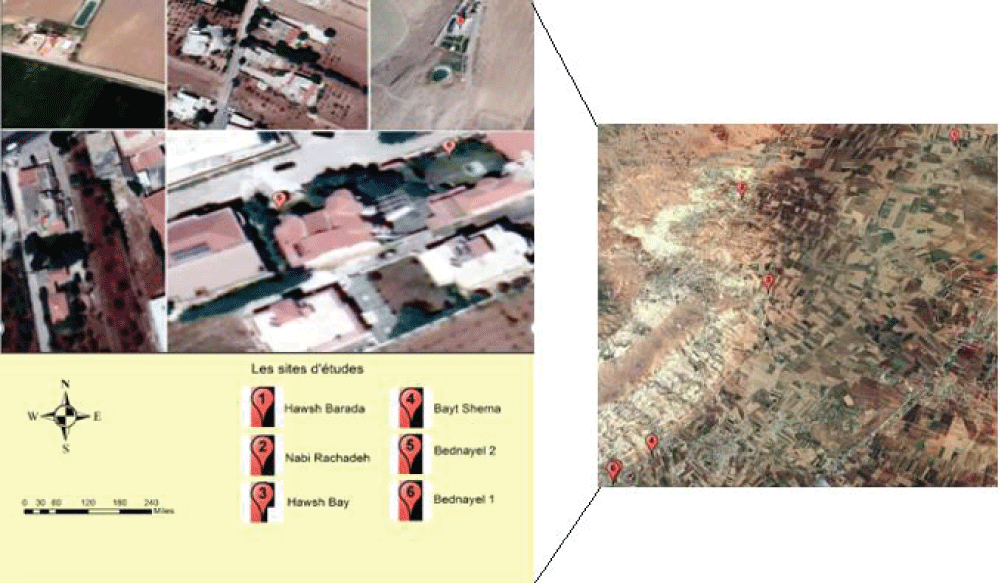
The sampled water was collected from several sources in Baalbek Region and its surrounding. The region occupies more than 300.000 people where agriculture is the main activity. In the area of concern, there are several water sources, in addition to the groundwater aquifers, water the Litani River and from a number of man-made lakes. The selected sites are distributed over several villages, and the distance between wells is almost at few kilometres, as these well were dug under a decision made by the Ministry of Energy and Water. There are six sites selected, these are: Bednayel, Nabi-Rashadah, Tamnine, Hawsh Barada, and Taraya (Hawsh Bay) and Beit Shama. Both domestic and agricultural uses of water exist. Table 1 and Figure 1 describes the types of activities presented around each site, as well as the geographic coordinates and altitude.
| Table 1: Geographic coordinates and activities of the sites studied | |||||
| Site | Name of village | Longitude | Latitude | Altitude (m) |
Type of activity |
| 1 | Hawsh barada | 36° 6'38.56"E | 34° 0'47.05"N | 1008 | Agriculture, Urban Area |
| 2 | Nabi Rashadah | 36° 2'18.38"E | 33°59'29.50"N | 1119 | Agriculture, Urban Area |
| 3 | Taraya (Hawsh bay) | 36° 3'0.49"E | 33°57'39.71"N | 1107 | Agriculture, Urban Area |
| 4 | Beit Shama | 36° 1'25.47"E | 33°55'6.16"N | 996 | Agriculture, Urban Area |
| 5 | Bednayel 1 | 36° 0'56.53"E | 33°54'45.17"N | 995 | Agriculture, Urban Area |
| 6 | Bednayel 2 | 36° 0'54.37"E | 33°54'43.75"N | 994 | Agriculture, Urban Area |
Figure 1: Map of sampling sites in the Eastern Bekaa region.
The samples were taken manually from all identified sources on June 2021. Hence, 18 bottles of 1.5 L capacity were used, each bottle corresponds to a target (well, water tank, tap), they are rinsed with first with sealed water bottles, after sampling, they are tightly closed with a well-glued cap to avoid any source of contamination. After labelling each bottle, the samples were stored in an isothermal refrigerator. Measurements were taken in-situ, in particular for the physicochemical parameters: pH, EC, TDS and in a short time, the water is tested in the water assessment laboratory at the Lebanese Agriculture Research Institute (LARI), the microbiology is tested first (3 times) to avoid bacterial multiplication, then the physicochemical parameters and the heavy metals. The techniques and devices that were used to evaluate the physicochemical parameters are (Table 2) including Spectrophotometry, Atomic absorption Spectrometry, Titrimetric method, Ion chromatography
| Table 2: The different physicochemical parameters measured and the analysis methods. | |||
| Parameters | Abbreviation | Unite | Analysis method |
| pH | pH | pH | Electrode |
| Conductivity | EC | µS/cm | Electrode |
| TDS | TDS | ppm | Electrode |
| Fluoride | F- | mg/l | Ion chromatography |
| Bromide | B- | mg/l | Ion chromatography |
| Nitrite | NO3- | mg/l | Ion chromatography |
| Hardness | D | mg/l | Titrimetric Method |
| Chloride | Cl- | mg/l | Titrimetric Method |
| Sulfate | SO42- | mg/l | Ion chromatography |
| Nitrate | NO3- | mg/l | Spectrophotometry |
| Phosphate | PO42- | mg/l | Spectrophotometry |
| Ammonium | NH4+ | mg/l | Spectrophotometry |
| Iron | Fe | mg/l | Spectrophotometry |
| Copper | Cu | mg/l | Atomic Absorption Spectrometry |
| Zinc | Zn | mg/l | Atomic Absorption Spectrometry |
| Chromium | Cr | mg/l | Atomic Absorption Spectrometry |
| Manganese | Mn | mg/l | Atomic Absorption Spectrometry |
| Cadmium | Cd | mg/l | Atomic Absorption Spectrometry |
| lead | Pb | mg/l | Atomic Absorption Spectrometry |
| Nickel | Ni | mg/l | Atomic Absorption Spectrometry |
Physio-chemical water analysis
For the pH, it determines the aggressive or stretching character of the water and it is a good indicator of water quality [13]. The values represented in Table 3 for the pH vary between 6.87 and 7.87 (all factors: physical, chemical and biological) can combine together to give a precise value of pH), which is within the limits recommended by LIBNOR (The Lebanese Standards Institution). Therefore, from an acid-base point of view, the water is acceptable as drinking water and does not cause harmful or cramps and other bodily abnormalities.
| Table 3: pH, EC and TDS results of the tests samples. | |||||
| Sites | Name of village | Source | pH | EC (µS/cm) |
TDS (g/l) |
| 1 | Hawsh barada | P | 7.18 | 0.60 | 0.29 |
| R | 7.29 | 0.65 | 0.33 | ||
| r | 7.42 | 0.75 | 0.37 | ||
| 2 | Nabi Rashadeh | P | 7.5 | 0.55 | 0.27 |
| R | 6.91 | 0.41 | 0.22 | ||
| r | 7.42 | 0.42 | 0.21 | ||
| 3 | Hosh bay | P | 7.59 | 0.45 | 0.22 |
| R | 7.86 | 0.32 | 0.17 | ||
| r | 7.87 | 0.33 | 0.16 | ||
| 4 | Beit shema | P | 6.87 | 0.58 | 0.26 |
| R | 7.16 | 0.60 | 0.30 | ||
| r | 6.94 | 0.63 | 0.32 | ||
| 5 | Bednayel 1 | P | 7.02 | 0.60 | 0.32 |
| R | 7.24 | 0.49 | 0.27 | ||
| r | 7.32 | 0.72 | 0.35 | ||
| 6 | Bednayel 2 | P | 6.96 | 0.58 | 0.31 |
| R | 7.41 | 0.53 | 0.24 | ||
| r | 7.31 | 0.48 | 0.23 | ||
| Limits recommended by LIBNOR (1999) | 6.5-8.5 mg/L | 1.5 µS/cm | 500 mg/L | ||
| *P = Well; R = tank and r = faucet. | |||||
The electrical conductivity (EC) values in relation to the mineralization are mentioned in Table 3, water contamination increases electrical conductivity. This parameter can be an indirect measure for Total Dissolved Solids (TDS) [14]. Hence, mineralization at all sites is considered very low. According to Table 3, the EC values vary between 0.32 and 0.75 µS/cm, which are acceptable according to LIBNOR in determining a maximum EC value of 1.5 µS/cm.
For the Total Dissolved Solids (TDS), as shown in Table 3, they show the variation in taste depending on the TDS. While, some samples have excellent palatability such as sites: 1P, 2P 2R 2r, 3P 3R 3r, 4P, 5R, 6R 6r where their TDS is less than 300 mg/L. All other sites have good palatability as their TDS varies between 300 mg/L and 600 mg/L. According to LIBNOR limits, the TDS values of all sites are acceptable since they do not exceed 500 mg/L; and therefore, the level of contaminants is not out of the limit.
Anions concentration in the selected sites
The concentration of anions in water (in mg/L) including the Ortho phosphate (PO43-), Fluoride (F-), Chloride (Cl-), Nitrite (NO2-), Nitrate (NO3-), Bromide (Br-), Sulfate (SO42-) are shown in Table 4, and they are among the limits recommended by LIBNOR.
| Table 4: Anions concentration in the selected sites. | ||||||||||
| Sites | Name of villages | Sources | PO43- | F- | Cl- | NH4+ | NO2- | NO3- | B- | SO42- |
| 1 | Hawsh barada | P | ND | 0.032 | 27.383 | ND | 0.004 | 145.99 | 0.207 | 9.732 |
| R | ND | 0.044 | 28.508 | ND | ND | 154.787 | 0.186 | 10.906 | ||
| R | ND | 0.052 | 28.435 | ND | 0.014 | 156.658 | 0.171 | 11.050 | ||
| 2 | Nabi Rashadeh | P | ND | 0.098 | 17.450 | ND | ND | 11.053 | 0.084 | 12.256 |
| R | ND | 0.149 | 17.359 | ND | 0.006 | 12.056 | 0.073 | 11.103 | ||
| R | ND | 0.085 | 16.037 | ND | 0.02 | 12.836 | 0.083 | 11.428 | ||
| 3 | Hosh bay | P | ND | 0.47 | 11.775 | ND | 0.003 | 8.582 | 0.023 | 11.924 |
| R | ND | 0.47 | 11.068 | ND | 0.004 | 9.260 | 0.033 | 11.551 | ||
| R | ND | 0.44 | 8.465 | ND | 0.002 | 12.792 | 0.024 | 8.719 | ||
| 4 | Beit shema | P | ND | 0.068 | 18.585 | ND | 0.007 | 39.118 | 0.062 | 18.425 |
| R | ND | 0.066 | 18.530 | ND | ND | 39.055 | 0.049 | 18.447 | ||
| R | ND | 0.063 | 21.205 | ND | 0.004 | 39.067 | 0.091 | 18.618 | ||
| 5 | Bednayel 1 | P | ND | 0.076 | 17.083 | 0.106 | 0.006 | 30.682 | 0.025 | 16.523 |
| R | ND | 0.110 | 16.607 | ND | 0.003 | 30.447 | 0.077 | 19.053 | ||
| R | ND | 0.092 | 19.697 | ND | ND | 31.115 | 0.035 | 18.750 | ||
| 6 | Bednayel 2 | P | ND | 0.048 | 19.690 | ND | 0.022 | 27.408 | 0.153 | 12.565 |
| R | ND | 0.043 | 19.446 | ND | ND | 5.592 | 0.068 | 11.487 | ||
| R | ND | ND | 18.723 | ND | 0.001 | 24.355 | 0.091 | 11.176 | ||
| Limits recommended by LIBNOR(1999) | 1 mg/L | 200 mg/L | 1.5 mg/L | 0.05 mg/L | 45 mg/L | 30 mg/L | 250 mg/L | |||
Ammonium, nitrite, and nitrate: It was noted that during the rainy season, ammoniac and nitrogen is adsorbed by soil, and it undergoes nitrification (in an oxidizing condition) as the groundwater piezometric level decreases and the unsaturated zone progresses [14-16]. While, the Nitrate is considered very mobile and quickly reaches the water table unlike ammonium [17,18]. The concentration of ammonium and nitrite is considered low at all sites and since they are below the threshold recommended by LIBNOR for drinking water (i.e., 1.5 mg/L for ammonium and 0.05 mg/L for nitrite). However, at site #1, the nitrate concentration is high and this is due to the use of fertilizers or the decomposition of infiltrated organic matter.
Chlorine: The chloride ion is characterized by the difficulty of adsorption and combination with rock lithologies, and by its high mobility. The mass concentration of chloride varies between 8,465 and 28,51 mg/L, thus it is considered lower than the standard recommended by LIBNOR which is 200 mg/L. This is due to the absence of industrial activity within the study area; therefore, it can be acceptable under the necessary conditions of drinking water. This low is attributed to least content of salt deposits (i.e., sodium chloride) in the existing rocks [17], the use of irrigation water with a low chloride content, the absence of industrial activities, namely the water with high chloride concentration are considered laxative and corrosive[18].
Sulfate: The sulfate concentration varies between 9,73 and 19,05 mg/L. By comparing these values with the limits recommended by LIBNOR, the sulfate level at all sites is acceptable, because they are less than 250 mg/L. This low concentration is due to several factors such as: A non-excessive use of sulfur-based fertilizers such as ammonium sulfate, insecticides and fungicides, the absence of industrial activities (mining industry, foundry, textile factories, etc.), a low abundance of sulfate-based minerals, the sulfate-reducing action of certain bacteria involved in the sulfur cycle [19].
Boron: The boron concentration at all sites is lower than the limit recommended by LIBNOR (0.023 - 0.0207 mg/L) which is 30 mg/L; hence, it is acceptable at all sites. Boron is found in water in the form of boric acid and its conjugate base, its low concentration is due to the absence of minerals such as tourmalines and datolites [19], its addition in agriculture is done in limited quantities as an essential trace element for the development of plants, in insecticides and fungicides.
Cations concentration in the selected sites
Water hardness: The hardness of water refers to the total concentration of the cations Ca2+ and Mg2+, the natural sources of hardness are the sedimentary rocks and mainly the carbonate ones (i.e., limestone and dolomite). The infiltration of water which passes through the carbonate rocks undergoes a dissolution (i.e., karstification) of calcium and magnesium. Water hardness values at the sites studied vary between 100 and 230.4 mg/L. The limit recommended by LIBNOR for drinking water is 250 mg/L, hence the hardness values are acceptable as shown in Table 5.
| Table 5: Cationic concentration in the selected sites in mg/L (ND: Not detected). | |||||
| Selected Sites | Sources | Hardness | Fe2+ | Ca2+ | Mg2+ |
| 1 | P | 230.4 | ND | 146 | 84.4 |
| R | 224.5 | ND | 142.5 | 82.42 | |
| r | 224 | ND | 143.2 | 80.7 | |
| 2 | P | 159 | 0.0522 | 100.75 | 58.2 |
| R | 155 | 0.0523 | 98.2 | 56.7 | |
| r | 155 | 0.0544 | 98.2 | 56.7 | |
| 3 | P | 100 | ND | 63.3 | 36.6 |
| R | 98 | 0.0033 | 62.1 | 35.9 | |
| r | 97 | 0.01000 | 61.4 | 35.5 | |
| 4 | P | 159 | 0.0588 | 100.75 | 58.2 |
| R | 157 | 0.0595 | 99.4 | 57.5 | |
| r | 157 | 0.0598 | 99.4 | 57.5 | |
| 5 | P | 173 | ND | 109.6 | 63.3 |
| R | 173 | ND | 109.6 | 63.3 | |
| r | 173 | ND | 109.6 | 63.3 | |
| 6 | P | 173 | 0.0189 | 109.6 | 63.3 |
| R | 171 | ND | 108.35 | 62.6 | |
| r | 170 | 0.0056 | 107.7 | 62.2 | |
| Limits recommended by LIBNOR (1999) | 250 mg/L | 0.3 mg/L | 200 mg/L | 13 mg/L | |
Calcium: Calcium comes from the attack of CO2 dissolved in water by limestone rocks such as dolomite [20]. The calcium level at the sites studied varies between 62 and 236 mg/L, the limit recommended by LIBNOR is 200 mg/L, site #5 has a calcium level of 236 mg/L which exceeds this standard limit, which makes this hard and non-drinkable water.
Magnesium: The magnesium level in the studied sites varies between 47 and 142 mg/L, while, the limit recommended by LIBNOR is 50 mg/L, site #5 has a magnesium level of 142 mg/L which exceeds this limit, which makes this water undrinkable.
Iron: Iron is an element present in rocks in a certain proportion, it is linked to silicates, oxides or hydroxides (e.g., hematite, magnetite, ferrihydrite), Sulphides or carbonates. Iron is found in water in the form of a ferrous ion and not a ferric one [20]. Generally, presence of iron in water comes down to the redox value of the medium. The quantity of ferrous ion present at the level of the tanks or at the level of the taps can be caused by: the use of tanks and metal pipes made not repaired over a long time. Corrosion occurs through the reaction between water, oxygen and iron to ultimately form rust. The results show very low corrosion represented by low quantity of ferrous ion, and this comes down to low concentrations of chloride, sulfates, ammonia, copper, manganese and a neutral pH (an acidic pH favors corrosion). A high level of dissolved oxygen increases oxidation. Iron, and soft water cannot protect the pipes from corrosion (by carbonate deposits). Comparing the ferrous ion results with that recommended by LIBNOR; hence, the iron content is acceptable because it is less than 0.3 mg/L.
Heavy metals
With the exception of zinc, which was found at sites #4 and 5, the concentration of heavy metals in wells is undetectable. The soil’s natural state can be restored if cadmium, lead, nickel, chromium, copper, and manganese are absent, as well as household waste is absent, fertilizers free of heavy metals (e.g., cadmium) are used, human activity is absent; especially in the case of nickel, and industrial activity is absent. The usage of phytosanitary chemicals may be the cause of the presence of a particular amount of zinc in wells [21] (Table 6).
| Table 6: Shows the heavy metals concentration is the selected sites. | ||||||||
| Sites | Sources | Zinc | Copper | Manganese | Chromium | Cadmium | Lead | Nickel |
| 1 | P | ND | ND | ND | ND | ND | ND | ND |
| R | ND | ND | ND | NDND | ND | ND | ND | |
| R | ND | ND | 0.0031 | NDND | ND | ND | ND | |
| 2 | P | ND | ND | ND | NDND | ND | ND | ND |
| R | 0.1181 | ND | ND | NDND | ND | ND | ND | |
| R | 0.4405 | 0.0469 | ND | NDND | ND | ND | ND | |
| 3 | P | ND | ND | ND | NDND | ND | ND | ND |
| R | ND | ND | ND | NDND | ND | ND | ND | |
| R | 0.0863 | 0.0339 | ND | NDND | ND | ND | ND | |
| 4 | P | 0.2169 | ND | ND | NDND | ND | ND | ND |
| R | 0.2188 | ND | ND | NDND | ND | ND | ND | |
| R | 4.0892 | ND | ND | NDND | ND | ND | ND | |
| 5 | P | 0.0237 | ND | ND | NDND | ND | ND | ND |
| R | 0.0851 | ND | ND | NDND | ND | ND | ND | |
| R | 1.3143 | 0.0469 | ND | NDND | ND | ND | ND | |
| 6 | P | ND | ND | ND | NDND | ND | ND | ND |
| R | ND | ND | ND | NDND | ND | ND | ND | |
| R | ND | ND | ND | NDND | ND | ND | ND | |
| Recommended limits by LIBNOR (1999) |
4 mg/L | 1 mg/L | 0.02 mg/L | 0.05 mg/L | 0.3 mg/L | 0.01 mg/L | 0.5 mg/L | |
| ND: Not detected. | ||||||||
The presence of zinc is noticeable in water tanks and faucets. The alloy of iron, carbon, and zinc used to make these tanks and pipes is called “galvanized steel”. There is no impact of neutral pH on pipes, while corrosion is encouraged by rising temperatures.
In water tanks and taps, we noticed the presence of zinc (4.082 mg/L). These tanks and pipes are made of an alloy of iron, carbon and zinc, that is to say “galvanized steel”. The temperature promotes corrosion when increasing. The presence of the copper ion can be the same causes of zinc corrosion, but it is considered less sensitive than zinc which is less sensitive than iron to corrosion [22,23].
Microbiological parameters
The results for the microbiological (bacteria) tests including the Total Coliforms (TC), Fecal Coliforms (FC), Streptococci and Pseudomonas in the water for the six sites studied are mentioned in Table 7.
| Table 7: Bacteria detected in the selected sites. | |||||
| Sites | Sources | CT or TC (UFC/100 ml 37 °C) |
CF (UFC/100 ml 45 °C) | Pseudomonas aeruginosa (UFC/100 ml 37 °C) | Streptococcus groupe D (UFC/100 ml 37 °C) |
| 1 | P | 145 | 0 | 0 | 0 |
| R | >200 | 0 | 0 | 0 | |
| R | >200 | 0 | 0 | 0 | |
| 2 | P | >200 | 5 | 3 | 3 |
| R | >200 | 55 | 44 | 30 | |
| R | >200 | 50 | 47 | 35 | |
| 3 | P | 70 | 0 | 0 | 0 |
| R | 130 | 3 | 0 | 0 | |
| R | 125 | 5 | 0 | 0 | |
| 4 | P | 140 | 0 | 0 | 0 |
| R | >200 | 0 | 0 | 0 | |
| R | >200 | 0 | 0 | 0 | |
| 5 | P | 0 | 0 | 0 | 0 |
| R | 0 | 0 | 0 | 0 | |
| R | 0 | 0 | 0 | 0 | |
| 6 | P | 0 | 0 | 0 | 0 |
| R | 0 | 0 | 0 | 0 | |
| R | 0 | 0 | 0 | 0 | |
| Recommended limits by LIBNOR 1999?) | 0 | 0 | 0 | 0 | |
Numerous factors, including water table depth, the suitability or lack of sanitary infrastructure, inadequate waste treatment, and the drawing technique, might contribute to well-induced water contamination [24]. Total Coliforms in wells: surface water, precipitation, and the close proximity of septic tanks to wells can all cause infiltration of residential waste water.
Fecal materials, such as manure, latrines, and septic tanks, are indicated by the presence of fecal coliforms and streptococci. Fecal pollution and well depth are the results of disregarding the distance between latrines and wells; contamination rises with decreasing water table [21]. Due to Streptococci’s high environmental resistance, contamination may still be present. Pseudomonas bacteria are highly resistant to environmental conditions and are found in soil and humid areas, particularly around taps and pipelines. The contamination increases with decreasing depth. Contamination by streptococci can be residual, because this bacteria resists well in the environment. The Pseudomonas bacteria is found in the soil and in humid environments, especially around taps and pipes, and is highly resistant to environmental conditions. An increase in the population of bacteria in the water tank and tap can result from the presence of bacteria on rusty sites in the pipes or in the form of biofilms.
The increase in temperature also causes bacterial colonies to proliferate [25]. Sites #2, 3 and #4 represent some corrosion which contributed to bacterial proliferation alongside the temperature, and the lack of hygiene from one period to another. Sites #5 and 6 are not contaminated, this comes down to respecting the distance between the septic tanks and the well, at a depth of the well preserving surface contamination, maintaining the hygiene of the tank from time to time. Other and the use of a filter at the tap. It can be concluded that sites #1, 2, 3, and 4 have water quality that is not acceptable from a microbiological point of view, exceeding the threshold recommended by LIBNOR (i.e., zero number of bacteria for drinking water), on the other hand, the Sites 5 and 6 have acceptable water, respecting the limit recommended by LIBNOR.
Correlation
The Pearson correlation coefficient, also known as the “correlation matrix”, is used to assess the variance of the water study characteristics by displaying how they vary from one another. The sources of every site (well, reservoir, tap) monitor the change in parameters (Table 8).
| Table 8: Correlation between parameters and sources of Contamination. | ||
| Site | Correlation Parameters | Sources of Contamination |
| 1 | Strong to moderate correlation between: nitrate, sulfate, fluoride, chloride and TC | Sediments: decomposition of organic micro-pollutants such as pesticides, bacteria growth site, sediments and organic matter in pipes, water stratification (stagnant water). |
| 2 | Strong to moderate correlation between: nitrite, TC, CF, Pseudomonas, Streptococci Zinc, CT, CF, Pseudomonas, Streptococci | Sediments or clay-humic complexes (denitrification process) or excretes of insects or plant debris. The increase in bacterial colonies increases bio-corrosion. |
| 3 | Strong to moderate correlation between: Nitrate, TC, CF Sediments (organic and inorganic micro-pollutants + source of bacteria). | Sediments (organic and inorganic micro-pollutants + source of bacteria). |
| 4 | Moderate correlation between: TC, sulfate, chloride Sediments | A portion of old water in the tank + stagnant water + polluted pipes. |
| 5 | Strong correlation between: chloride and nitrate zinc and copper | A portion of old????? water + presence of sediments deposits + stagnant water pipe corrosion. |
Principal component analysis (PCA)
Principal Component Analysis (PCA) is a multivariate analysis method allowing the simultaneous study of a large number of variables whose total information cannot be visualized because of a space with more than three dimensions. This method would make it possible to specify the relationships between the variables and the phenomena at the origin of these relationships. The objective is to have information concentrated on a minimum of axes. It is widely used to interpret hydro-chemical data. Therefore, PCA is a tool that offers the possibility of simplifying the study of aquatic ecosystems and reducing costs by reducing the number of variables to take into account.
Choice of factorial axes by Kaiser Criterion: The Kaiser criterion method consists of retaining the axes having an eigenvalue λ > 1. According to the table above, we have three eigenvalues greater than 1 (λ1 = 5.984, λ2 = 1.562, λ3 = 1.204), so this criterion recommends taking the first three axes.
These axes represent a total inertia (explanation of parameters) is equal to 87.50% ≈ 88%, of which axis 1 expresses 59.9% and axis 2 expresses 15.6%, and axis 3 expresses 12.0%.
Choice of active variables: Table 9 gives, in the Extraction column, the percentage of information from each of the 10 variables (i.e., parameters) which is reproduced by the model. It displays the extraction value of each physicochemical parameter in our study, from where it measures the quality of representation in the principal component analysis for each.
| Table 9: Choice of factorial axes. | |||
| Eigen value | Percentage of variance | Cumulative percentage | |
| comp 1 | 5.984 | 59.838 | 59.838 |
| comp 2 | 1.562 | 15.624 | 75.462 |
| comp 3 | 1.204 | 12.043 | 87.504 |
| comp 4 | .584 | 5.837 | 93.341 |
| comp 5 | .442 | 4.424 | 97.765 |
| comp 6 | .101 | 1.012 | 98.777 |
| comp 7 | .089 | .889 | 99.666 |
| comp 8 | .033 | .331 | 99.997 |
| comp 9 | .000 | .003 | 100.000 |
Furthermore, it is clear that all the physicochemical parameters in our data are representative because all the extraction values exceed 0.6, and their values are close to the initial value (1)(Table 10).
| Table 10: Active parameters variables. | ||
| Initial | Extraction | |
| F- | 1.000 | 0.759 |
| Cl- | 1.000 | 0.945 |
| NO2- | 1.000 | 0.816 |
| NO3- | 1.000 | 0.848 |
| B- | 1.000 | 0.904 |
| SO42- | 1.000 | 0.779 |
| Hardness | 1.000 | 0.984 |
| Fe2+ | 1.000 | 0.746 |
| Ca2+ | 1.000 | 0.985 |
| Mg2+ | 1.000 | 0.984 |
Coordinate matrix
We can deduce from the coordinate table (Table 11) the variables which are well represented and contribute to the construction of each axis:
Axis 1: F-, Cl-, NO3-, B-, Hardness, Ca2+, Mg2+
Axis 2: SO42-, Fe2+
Axis 3: NO2-, SO42-, Fe2+
| Table11: Coordinate Table of parameters. | |||
| Parameters | Axe 1 | Axe 2 | Axe 3 |
| F- | -.722 | -.486 | -.039 |
| Cl- | .969 | .053 | -.058 |
| NO2- | .162 | .021 | .888 |
| NO3- | .869 | -.289 | -.102 |
| B- | .888 | -.219 | .262 |
| SO42- | -.078 | .795 | -.374 |
Correlation circle: Each cloud of points (i.e., variables and individuals) is constructed in projection onto the factorial planes; a factorial plan is a benchmark of the plan defined by two of the q factorial axes retained. If we retain 3 axes, then 2 graphs will be drawn for each cloud: the cloud projected on the plane (axis1, axis2), plane (axis1, axis 3), plane (axis 2, axis 3).
Examining the factorial designs will make it possible to visualize the correlations between the variables and to identify groups of individuals who have taken close values on certain variables.But before reading the graphs directly, the axes must be interpreted and ensure that the projection is faithful to reality.
In ACP two types of factors present themselves:
- Size effect: The variables are all on the same side of the axis. (i.e. they all contribute in the same direction to the formation of the axis).
- Shape effect: Two groups of opposing variables: those which contribute positively to the axis, those which contribute negatively.
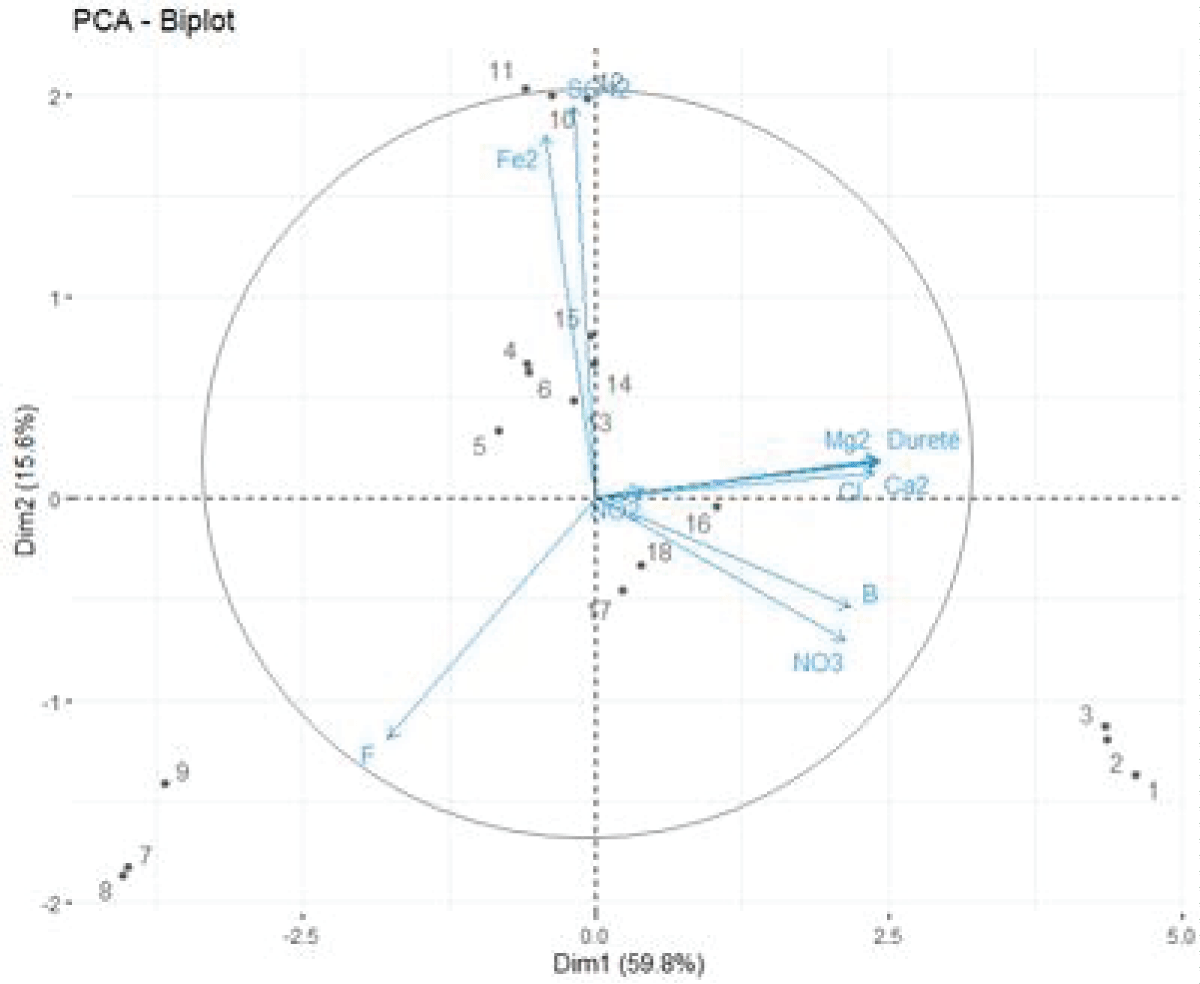
Figure 1 gives us useful and necessary information to understand and analyse the different variables used in this thesis, and which are distributed in the 4 quadrants of the Figure.
The PCA diagram for parameters and individuals on axes 1 and 2 explains ≈ 75.4% of the information and it has great importance.
For parameters
From the Figure 2 (i.e., correlation circle), we can see that all parameters except (NO2-) are very close to the correlation circle and therefore very well represented on the PCA diagram. The rather closed angle (starting from the origin) formed by the parameters: NO2-, Mg2+, Cl-, Ca2+, Hardness indicates that they are very well correlated with each other, and the same for the parameters: Fe2+ and SO42-, and for the two parameters: NO3- and B-.Figure 2: Diagram of the variables of individuals in space after rotation on axes 1 and 2.
Again we can notice the almost right angle formed by the parameters: NO2-, Mg2+, Cl-, Ca2+, respectively with the parameters Fe2+ and SO42-, indicates that they are independent of them. In addition, the parameter F- again is independent of all parameters.
For individuals (well, reservoir, tap)
-The sources of each site are contaminated and affected by chemical pollutants in an almost similar way (in similar quantities) as Figure 2 shows.
-Each site is affected differently by chemical pollutants thanks to the difference in sources of contamination from the well to the tap [26-46].
Water sources are continuously exposed to pollution, which can manifest in physico-chemical, microbiological, or mixed forms, adversely affecting water quality. Contamination can originate from natural sources or human activities. This study focuses on the variation in water quality, especially within a complex distribution system that can impact water parameters, potentially causing diseases among rural communities sometimes with fatal outcomes. The water distribution system in this study includes irrigation canals, water tanks, and pumps. Due to various environmental factors, this system can degrade water quality, making it undrinkable.
Due to various environmental conditions, this system can affect the quality of the water and make it undrinkable. Six sites were studied to assess the quality of well water and examine all the factors that can affect it from the well to the tap after being put in reserve, and finally investigate if this water is suitable as drinking water. Hence, the physicochemical parameters (pH, TDS, EC, kkNO2-, SO42-, Fe2+, F-, B-, SO42-, Cl-, Mg2+, NH4+, Ca2+, Hardness, Zn, Cd, Cu, Pb, Mn, Ni, Cr), and microbiological (Staphylococcus aureus, CT, Escherichia coli, Pseudomonas aeruginosa) were evaluated.
The results show that the physicochemical factors at sites #2, 3, 4, 5, and 6 comply with the standards recommended by LIBNOR for drinking water. However, Site #1 is excluded due to the detection of high nitrate levels in its three sources, likely due to intensive agricultural activity. The concentration of cations and anions varies across the six sites, with contaminants being more concentrated at some sites, as indicated by the PCA results.
This can be attributed to the location of the well, its depth, its vicinity (e.g., domestic waste, anthropogenic activities, etc.), the present of rock lithologies, and the degree of hygiene.
Regarding heavy metals, only sites #4 and 5 present a very small quantity at the wells, and all other heavy metals are undetected, due to the almost zero presence of industrial activities. Iron is present in very low quantities at sites #2, 4 and 6 in the well. Tank and tap water are affected by the same well contaminants, without significant change in quality from a physicochemical properties. A presence of a small quantity of heavy metals at the level of the tanks and the tap such as zinc in sites #2, 3, 4, 5 with copper at the tap of sites #2 and 5 which amounts to corrosion, has the value of zinc increases from tank to tap, but it meets the LIBNOR standard. For microbiological analysis, we noticed an increase in contamination by bacteria going from the well to the tap at sites #1, 2, 3 and 4. While, site #3 presents all types of bacteria studied.
Therefore, we can say that bacterial contamination undergoes a dangerous proliferation from the well to the tap by exceeding the limited threshold. We still notice the appearance of fecal contamination at site #3 which is absent at the well level. Sites 5 and 6 are respectable by a zero value of all bacteria in all sites. So we can conclude that sites #1, 2, 3, 4 have non-drinkable water, but sites #5 and 6 are acceptable and have drinking water.
Iron is present in very low quantities at sites #2, 4, and 6. The water in tanks and taps reflects the same well contaminants, with no significant change in physicochemical properties. However, small amounts of heavy metals, such as zinc at sites #2, 3, 4, and 5 and copper at taps in sites #2 and 5, are detected, likely due to corrosion. The concentration of zinc increases from the tank to the tap, though it remains within LIBNOR standards.
For microbiological analysis, there is an observed increase in bacterial contamination from the well to the tap at sites #1, 2, 3, and 4, with site #3 showing the presence of all studied bacteria types. Bacterial contamination proliferates dangerously from the well to the tap, exceeding safety thresholds. Additionally, fecal contamination is detected at site #3, absent at the well level. Sites #5 and 6 are free of all bacteria across all stages, making their water safe for drinking. In conclusion, water from sites #1, 2, 3, and 4 is non-potable, while sites #5 and 6 provide drinkable water.
Overall, water quality primarily depends on the well’s condition and the entire distribution system. From a physico-chemical perspective, water quality variation is minimal, except for heavy metals, which are mainly influenced by the distribution system’s interaction with various factors. In contrast, poor hygiene practices lead to increased bacterial growth from the well to the tap.
In order to improve water quality and conserve human and the environment in the Baalbek Region, the following are recommended:
- Maintain disinfectants such as chlorine or chloramine throughout the distribution system.
- Attention must be given to the age of the water in your system: The age of the water in the tank should not exceed 5 to 7 days to avoid developing bacteria like legionella.
- Regularly inspect of storage facilities (detect openings and cracks in the system) is a must.
- Maintain the system pressure (i.e., p > 35 psi) to avoid water return problems.
- Monitor interconnections and implement it.
- Flush the system from one period to another.
- Have standard operating procedures for all your routine and emergency procedures.
- Establish standard operating procedures for routine and emergency operations, including preventive maintenance programs such as valve exercising and fire hydrant inspection and maintenance.
- Postel SL, Daily GC, Ehrlich PR. Human appropriation of renewable fresh water. Science. 2008;271(5250):785-788. Available from: http://www.jstor.org/stable/2889886
- Saab HB, Nassif N, El Samrani AG, Daoud R, Medawar S, Ouaïni N. Suivi de la qualité bactériologique des eaux de surface (rivière Nahr Ibrahim, Liban). Rev Sci Eau. 2008;20(4):341-352. Available from: https://doi.org/10.7202/016909ar
- Awad S. Hydrochimie et faciès géochimiques des eaux souterraines, Plaine de Bekaa. Hydrol Sci J. 2011;56(2):334-348. Available from: http://dx.doi.org/10.1080/02626667.2011.559331
- Kassem Y, Gökçekuş H, Aljamal J. Surface water resource and effect of weather parameters in estimating the annual rainfall: a case study in Lebanon. IOP Conf Ser Mater Sci Eng. 2020;800(1). Available from: https://iopscience.iop.org/article/10.1088/1757-899X/800/1/012028
- Bengarnia Benmerine. Contribution to the study and evaluation of the physical-chimique and bacterial quality of the consumption products of the Oued Es-Saoura region. 2020.
- Nehme N, Mousa Haidar C, Dib A, Azouj N, Tarawneh K. Quality assessment of groundwater in the lower Litani Basin (LLRB), Lebanon. Geosciences Research. 2020;5(1). Available from: http://dx.doi.org/10.22606/gr.2020.51001
- Haidar C. Evaluation of the quality of the water of the lower bassinet of the river du Litani, Liban: environmental approach [Internet]. 2018. Available from: HAL Id: tel-01751390
- Nehme N, Mousa Haidar C, Rammal M, Abou Abbas F, Rammal H, Azouj N. Study of the water assessment in the Alhoujair Valley in Lebanon. J Mater Sci. 2021;12(11):1392-1404. ISSN: 2028-2508. Available from: https://www.jmaterenvironsci.com/Document/vol12/vol12_N11/JMES-2021-12112-Nehme.pdf
- Diab W, Haidar CM, Farhat M, Rammal M, Awad A, Hamzeh A. Drinking-water quality assessment in selective schools from Mount Lebanon. Ann Civil Environ Eng. 2024;7(8). Available from: https://doi.org/10.29328/journal.acee.1001061
- Nehme N, Mousa Haidar C, AL-Jarf Z, Abou Abbas F, Moussa N, Youness G, Tarawneh K. Assessment of the physicochemical and microbiological water quality of Al-Zahrani River Basin, Lebanon. Jordan J Earth Environ Sci. 2021;12(3):206-213. Available from: https://hal.science/hal-03295552
- Nehme N, Haydar C, Koubaissy B, Fakih M, Awad S, Toufaily J, Villieras F, Hamieh T. Metal concentrations in river water and bed sediments of the Lower Litani River Basin, Lebanon. J Adv Chem. 2014;8(2):1590-1601. Available from: http://dx.doi.org/10.24297/jac.v8i2.4040
- MDH M. Physico-chemical, bacterial and impact characteristics on surface eaux and souterraines, BAMAKO-Mali [Internet]. 2005;1-135. Available from: https://www.scirp.org/reference/referencespapers?referenceid=2517539
- World Health Organization. Guidelines for drinking-water quality. 4th ed. Geneva: World Health Organization; 2017. Available from: https://www.who.int/publications/i/item/9789241549950
- Nehme N, Haidar C. The physical, chemical, and microbial characteristics of Litani River water. In: The Litani River Lebanon: an assessment and current challenges. Water Science and Technology Library. Springer; 2018;57. Available from: http://dx.doi.org/10.1007/978-3-319-76300-2_4
- Ambroise B. The dynamique of the cycle of the water in a versatile bass: processus, facteurs, modèles. Bucharest, Romania: Editions HGA; 1999;206. Available from: https://search.worldcat.org/title/dynamique-du-cycle-de-leau-dans-un-bassin-versant-processus-facteurs-modeles/oclc/490958974?referer=di&ht=edition
- Metahri S. Simultaneous elimination of air pollution and phosphaté from the eaux used treated by the proper procedures. 2012;172. Available from: https://dspace.ummto.dz/items/7c17bbda-cf82-495f-a4a0-8d481dc7b5b1
- Laigle D, Kugler J, Kobus H, Zilliox L. Contamination des eaux souterraines par les nitrates: recherches et applications dans le cadre de la coopération franco-allemande. Hydrogeologie. 1990;1:51-160. Available from: https://core.ac.uk/download/pdf/147554441.pdf
- Ayad W, Kahoul M. Evaluation of the physico-chimique and bactériologique quality of the southern waters: case of the puits of the El-Harrouch region (Wilaya de Skikda). J Mater Environ Sci. 2016;7:1288-1297. Available from: https://www.jmaterenvironsci.com/Document/vol7/vol7_N4/143-JMES-2017-Ayad.pdf
- Viennot P. La pollution du bassin de la Seine par les nitrates [Internet]. 2009. Available from: www.eau-seine-normandie.fr
- Akil A, Hassan T, El Hamichi FP, Lahcen B, Abderrahim L. Study of the physical-chemical quality and metal contamination of the surface eaux of the bassin versant de Guigou, Maroc. Eur Sci J. 2014;10(23):1857-1881. Available from: https://core.ac.uk/download/pdf/236415782.pdf
- Soncy K, Djeri B, Anani K, Eklou-Lawson M, Adjrah Y, Karou D, et al. Evaluation of the bacterial quality of eaux de puits et de forage in Lomé, Togo. J Appl Biosci. 2015;91(1):8464. Available from: http://dx.doi.org/10.4314/jab.v91i1.6
- Ramírez E, Rios JF, Calderón JA, Echeverría F, Peñuela G. Alteration of the drinking water quality by different metallic materials used in distribution systems. Corros Prot. 2009;1(4):1443-1450. Available from: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=05ebed9fd90d42dc09b61a137c03d1a589769443
- Nehme N, Haidar CM, Diab W, Tarawneh K, Villieras F. Assessment of heavy metal pollution in the sediments of the Lower Litani River Basin, Lebanon. Jordan J Earth Environ Sci. 2019;10(2):104-112. Available from: https://www.researchgate.net/publication/335820473_Assessment_of_Heavy_Metal_Pollution_in_the_Sediments_of_the_Lower_Litani_River_Basin_Lebanon
- MEDSTAT-Environnement RL. Compendium statistique national sur les statistiques de l’environnement au Liban 2006. Administration Centrale de la Statistique [Internet]. 2006;(2):1-38. Available from: http://www.cas.gov.lb/images/PDFs/enviromment.pdf
- Eau P-CDEL, La CASDE. Contribution to the evaluation of the physical and chemical quality of the water: case of the urban river Gombe de Kinshasa/République Démocratique du Congo. 2016;26:7-29. Available from: https://openurl.ebsco.com/EPDB%3Agcd%3A15%3A14063503/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A116581987&crl=c
- Amir B, Omar B, Yasmina M. Elimination of industrial pollutants by adsorption on carbon dioxide and photocatalytic degradation on TiO2. J Mater Environ. 2011;2011. Available from: https://dspace.univ-ouargla.dz/jspui/handle/123456789/8788
- DGS. L’eau potable en France, 2002-2004. Guide Tech Eau Santé [Internet]. 2005;53. Available from: https://sante.gouv.fr/
- Ohno T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol. 2002;36(4):742-746. Available from: https://doi.org/10.1021/es0155276
- Banas D, Lata JC. Les phosphates. Le livre blanc des polluants de l’habitat [Internet]. 2006;121(2).
- Chery L, Barbier J. Le phosphore dans les eaux souterraines en France. État des connaissances. Année 1. 2000;2000:63. Available from: https://infoterre.brgm.fr/rapports/RR-40857-FR.pdf
- Akil A, Hassan T, El Hamichi FP, Lahcen B, Abderrahim L. Study of the physical-chemical quality and metal contamination of the surface eaux of the bassin versant de Guigou, Maroc. Eur Sci J. 2014;10(23):1857-1881. Available from: https://oa.mg/work/1902432142
- Garcia J, Roger P. Le cycle du soufre. 2000;1-29.
- National Agency for Food, Environmental and Occupational Health Safety (ANSES). Sheet 9: Assessment of health risks linked to exceeding the quality reference for sulfates in water intended for human consumption. Analytical uncertainty 4- Exposition. 2005;108-35.
- Lamprey-Maldonado DK. Characterization and origin of métaux traces, aromatic hydrocarbons, polycyclics and pesticides transported by the atmospheric retombées and ruisellement eaux in the bassins versatile separatifs péri-urbains [Internet]. 2009;297. Available from: https://theses.hal.science/tel-00596847
- Salvarredy Aranguren MM. Contamination in métaux lourds des surface eaux et des sediments du Val de Milluni (Bolivian Andes) par dechets miniers: approches géochimique, minéralogique et hydrochimique. 2008;489. Available from: https://theses.hal.science/tel-00277431
- Serpaud B, Casteignau M, Matejka G. Adsorption des métaux lourds (Cu, Zn, Cd et Pb) par les sédiments superficiels d’un cours d’eau: rôle du pH, de la température et de la composition du sédiment. Revue des sciences de l’eau. 1994;7(4). Available from: https://www.erudit.org/fr/revues/rseau/1994-v7-n4-rseau3277/705205ar.pdf
- Ombeni J, Munyuli TM, Lwango A, Mwangi T, Nabintu F, Izuba E, et al. Bacteriological quality of street foods vended in Bukavu City: potential health risks to consumers. Bact Emp. 2018;1(1):13-21. Available from: https://office.scicell.org/index.php/BE/article/view/12
- Escherichia coli dans l'eau potable. 2019. Available from: https://www.canada.ca/content/dam/hc-sc/documents/programs/consultation-e-coli-drinking-water/document/e-coli-consultation-FRA-June-10-2019.pdf
- Anonyme 3. Les faits en eau potable: bactéries coliformes, coliformes totaux et E. coli. 2017;4. Available from: https://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/fr/MilieuxSains/eau/Coliformf.pdf
- Santé Canada. Recommandations pour la qualité de l’eau potable au Canada: document technique - cadmium [Internet]. 1986. Available from: https://www.canada.ca/fr/sante-canada/services/publications/vie-saine/recommandations-pour-qualite-eau-potable-canada-document-technique-cadmium.html#s5_2
- Côté C. Risk of microbiological contamination of soil waters and preventive measures for adopter. 2004.
- Gauthier MF. Biofilms and biological quality of drinking water. Thèse. 2002.
- R-N. Modélisation de la corrosion des conduites d’eau potable en fonte de la ville de Québec. 2012.
- Bensaada S. Corrosion courses. 2017;79. Available from: https://www.univ-biskra.dz/enseignant/bensaada/corrosion.pdf
- Wang W, Du C, Chen N, Chen Z, He X. Spatial distribution, sources identification, and risk of the trace metals in surface sediments of Chaohu Lake using multivariate statistics and geostatistics. Nat Environ Pollut Technol. 2017;16(2):339-350. Available from: https://neptjournal.com/upload-images/NL-60-3-(1)D-605com.pdf
- Rubio B, Nombela MA, Vilas F. Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull. 2000;40(11):968-980. http://dx.doi.org/10.1016/S0025-326X(00)00039-4