More Information
Submitted: November 01, 2024 | Approved: November 12, 2024 | Published: November 13, 2024
How to cite this article: Ortega MET, Hernández SE, Hernández MEG, Figueroa RB, Castañeda FM, Zermeño CIV, et al. Comparing Immunity Elicited by Feedback and Titered Viral Inoculation against PEDV in Swine. Insights Vet Sci. 2024; 8(1): 028-038. Available from: https://dx.doi.org/10.29328/journal.ivs.1001044.
DOI: 10.29328/journal.ivs.1001044
Copyright License: © 2024 Ortega MET, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Coronavirus; Porcine; Epidemic; Diarrhea; Swine; Feedback; Immunity
Comparing Immunity Elicited by Feedback and Titered Viral Inoculation against PEDV in Swine
María Elena Trujillo Ortega1  , Selene Fernández Hernández2, Montserrat Elemi García Hernández2
, Selene Fernández Hernández2, Montserrat Elemi García Hernández2 , Rolando Beltrán Figueroa1
, Rolando Beltrán Figueroa1 , Francisco Martínez Castañeda3
, Francisco Martínez Castañeda3 , Claudia Itzel Vergara Zermeño1, Sofía Lizeth Alcaráz Estrada4
, Claudia Itzel Vergara Zermeño1, Sofía Lizeth Alcaráz Estrada4 , Elein Hernández Trujillo5
, Elein Hernández Trujillo5 and Rosa Elena Sarmiento Silva2*
and Rosa Elena Sarmiento Silva2*
1Department of Pig Medicine and Animal Husbandry, Faculty of Veterinary Medicine and Animal Husbandry, National Autonomous University of Mexico, Ciudad Universitaria, Av. Universidad #3000, Mexico City 04510, Mexico
2Department of Microbiology and Immunology and ELDORADO, Faculty of Veterinary Medicine and Zootechnics, National Autonomous University of Mexico, Ciudad Universitaria, Av. Universidad #3000, Mexico City 04510, Mexico
3Institute of Agricultural and Rural Sciences, Autonomous University of the State of Mexico, Literary Institute # 100, Col. Centro, Toluca 50000, Mexico
4Division of Genomic Medicine and Clinical Genetics, National Medical Center “20 de Noviembre”, Institute of Security and Social Services of State Workers, Av. Félix Cuevas #540, Mexico City 03100, Mexico
5Department of Clinical Studies and Surgery, Faculty of Higher Studies Cuautitlán, National Autonomous University of Mexico, Km 2.5 Cuautitlán-Teoloyuca Highway, Cuautitlán Izcallli 54714, Mexico
*Address for Correspondence: Rosa Elena Sarmiento Silva, Department of Microbiology and Immunology and ELDORADO, Faculty of Veterinary Medicine and Zootechnics, National Autonomous University of Mexico, Ciudad Universitaria, Av. Universidad #3000, Mexico City 04510, Mexico, Email: [email protected]
Porcine Epidemic Diarrhea Virus (PEDV) can infect pigs of any age, but the disease severity varies significantly, particularly affecting neonatal piglets due to their immature immune system. Various vaccination strategies have been questioned for their efficacy, especially since outbreaks have occurred even on vaccinated farms. Recent suggestions indicate that exposure to the virus may enhance the effectiveness of inactivated vaccines, highlighting the potential benefits of using attenuated viruses to generate immunity in sows without prior exposure. This study aimed to evaluate the humoral and cytokine responses in pregnant sows and their piglets after inoculation of affected piglet intestinal contents and a virus isolated. We measured immune parameters such as IL-12, IL-22, IgG, and IgA, as well as neutralizing antibodies in serum, colostrum, and milk. Notably, higher titers of neutralizing antibodies were found in sows immunized with the viral inoculum, while IL-12 and IL-22 levels showed no significant differences. Additionally, we assessed productive parameters like total piglets born, weaning mortality, average birth weight, and stillborn rates. The results indicated that sows treated with affected piglet intestinal contents had higher mortality (48.31%) and stillborn rates (20.96%) compared to those receiving the isolated virus (30.02% and 10.44%, respectively). These findings suggest that using an isolated virus can offer a safe, long-lasting, and specific immune response, underscoring the importance of thorough analysis of both systemic and mucosal immune responses against PEDV.
The Porcine Epidemic Diarrhea Virus (PEDV) is a member of the family Coronaviridae, genus Alphacoronavirus, subgenus Pedacovirus. It consists of enveloped viruses with a 28 kb single-stranded positive-sense RNA genome [1,2]. PEDV causes severe enteritis, characterized primarily by diarrhea, often accompanied by vomiting, anorexia, dehydration, and weight loss [3]. The PEDV was first reported in 1971 and isolated during an outbreak in Belgium in 1976 [4]. In 2013 a Porcine Epidemic Diarrhea (PED) outbreak occurred in Iowa, United States of America, followed by outbreaks in Mexico and Canada [3,5].
In Asia, PEDV infections result in significant economic losses, and vaccination using attenuated or inactivated vaccines produced from reference strains has been used for several years [6]. In America, a variety of vaccination strategies, including inactivated, attenuated, and truncated spike genes, have been used. However, their efficacy has been questioned due to the need for prior exposure and the occurrence of repeated outbreaks on vaccinated farms [7]. Lee, et al. in 2018 suggested that exposure to the virus is necessary to enhance the effectiveness of inactivated virus vaccines, proposing the usefulness of a controlled dose of attenuated virus to generate immunity in sows without prior exposure [8]. During PEDV outbreaks in underdeveloped regions, it has been common to immunize animals using the intestines of infected piglets, a practice known as ‘feedback’ [6]. However, this method carries the risk of iatrogenic infections that can adversely affect productive parameters, as well as the potential for failure due to uncontrolled viral titers in the samples [9].
Significant research has focused on improving PEDV prevention and treatment; Geiger & Connor (2013) identified feedback as the preferred treatment for breeding sows as a practical approach to stimulate the immune system in females, thereby facilitating passive immunity transfer to piglets via colostrum. However, when using this practice, the quantity of infectious virus inoculated into sows is unknown, and the immune response generated has not been monitored, nor have other potential pathogens been evaluated in the samples used [10].
Like other coronaviruses, various PEDV variants exhibit different degrees of virulence. S-INDEL strains are associated with less severe infections, characterized by a longer incubation period, shorter diarrhea duration, localized intestinal damage, and lower mortality (18% compared to 55% for NO-INDEL strains) [11]. While S-INDEL strains have been proposed as possible immunogenic candidates, cross-protection between these strains is only partial and often strain-specific. The degree of protection depends on the genetic similarity between strains and the immune response generated by the initial infection or vaccination, making an understanding of cross-protection essential for effective vaccine development [12-14].
PEDV can infect swine of all ages, but the severity of the disease is largely dependent on the animal’s age, with neonates being especially susceptible and facing high mortality [15]. The transfer of passive immunity from sows to piglets is essential for reducing mortality due to the high virulence of PEDV and the immature immune systems of young pigs. Studies of Transmissible Gastroenteritis Virus (TGEV), have shown litter protection, associated with high levels of antibodies in the sow’s milk, independent of maternal serum antibodies [16]. Lactogenic immunity is vital for defending neonates against enteric pathogens, and maternal immunization offers benefits for both the mother and her piglets. An enhanced maternal immune response during gestation and lactation leads to increased IgA levels in milk improving piglet protection until about 13 days of age [17]. Additionally, the method of vaccine administration plays a significant role, with oral vaccines producing the highest levels of IgG/A in the milk [18,19].
Inflammation mediated by the innate immune system is an important indicator of host disease resistance. Cytokines are the key modulators for various homeostatic and inflammatory processes. The interleukin-12 (IL-12) family plays important regulatory roles in immune response against infectious and autoimmune diseases, as well as cancer [20-22]. IL-12 is particularly relevant in viral infections, promoting cellular immunity by inducing the differentiation of naïve T cells into Th1 cells and B cells [20]. IL-12 also facilitates the development of secretory IgA (S-IgA) at mucosal surfaces and mediates inflammatory reactions by inducing antiviral cytokines such as IL-18, which recruits innate immune cells to infection sites [23].
IL-22 also plays a crucial role in controlling infections and maintaining mucosal integrity [24]; It promotes the expression of mucin genes, increases goblet cells, and enhances mucus production. IL-22 has protective functions in the intestine, reducing pathogen adherence and influencing the physiopathology of diarrhea by upregulating the claudin-2 protein, which regulates epithelial junctions. Its stimulation can lead to water loss and subsequent diarrhea [25,26]. Recent studies have shown that recombinant IL-22 broadly inhibits the replication of enteric coronaviruses (PEDV and TGEV) and porcine rotavirus in vitro [27,28]. While the precise mechanism of IL-22 antiviral activity is not fully understood, it has been shown to induce the expression of antimicrobial peptides and, in synergy with IFNƛ contributes to reducing the viral infection in intestinal cells [24].
In this study, we evaluated the humoral and cytokine responses in pregnant sows and their piglets after inoculation with affected piglet intestinal contents and an isolated virus. We measured immune parameters such as IL-12, IL-22, IgG, and IgA, as well as neutralizing antibodies in serum, colostrum, and milk. Notably, higher titers of neutralizing antibodies were found in sows immunized with the viral inoculum, while IL-12 and IL-22 levels showed no significant differences. Additionally, we assessed productive parameters like total piglets born, weaning mortality, average birth weight, and stillbirth rates. Our results indicated that sows treated with affected piglet intestinal contents had higher mortality (48.31%) and stillbirth rates (20.96%) compared to those receiving the isolated virus (30.02% and 10.44%, respectively).
Ethical statement
The project was carried out in the Porcine Production Teaching, Research, and Extension Center of the Faculty of Veterinary Medicine and Zootechnics of the National Autonomous University of Mexico (Centro de Enseñanza, Investigación y Extensión en Producción Porcina, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México), located at Km 2 of the Jilotepec-Corrales highway in Jilotepec, Estado de México. This is a 150-sow farrow-to-finish farm with self-replacement practices. The farm averages 8 births per week. Piglets are weaned at 21 days, moved to growth pens for five weeks, and then fattened until the end of the production cycle. During the study, the farm exhibited clinical signs suggestive of PED. Confirmatory tests were done by immunochromatography (anigen Rapid PED TEST KIT/RG14-01, Bionote) and a commercial RT-PCR (VetMAX™ PEDV/TGEV/SDCoV Kit, Applied Biosystems™ by Thermo Fisher Scientific). The study was evaluated and approved by the FMVZ-UNAM ethics committee (SICUAE. MC-2020/4-4). The animals were monitored daily to ensure their welfare and humane endpoints were determined prior to the start of the experiment to prevent unnecessary animal pain; euthanasia methods were considered according to AVMA Guidelines for the euthanasia of animals and NOM-033-SAG/ZOO-2014.
Viral stock
PEDV was propagated as described by Trujillo, et al. 2016 [5]. Briefly, Vero cells were inoculated with PEDV, strain MX.EdoMexC2A1S_2014, No-Indel strain GII (GenBank KM044335.1) [29] in D-MEM supplemented with 10 μg/ml of Trypsin (Gibco ™ Trypsin Thermo Fisher 250 Cat. 215240, Lot. 4181462, USA). At 48 h post-infection, the cells were resuspended, collected, and sonicated for 5 min at 37 °C (Bransonic Sonifier, 5510 Ultrasonic cleaner, Thermo Fisher, USA), and centrifuged at 5000 rpm for 10 min. Supernatant titrations were performed with TCID50/mL, using the Reed & Münch method. The inoculum used for the treatment contained 1X108 TCID50/mL.
Study groups
Pregnant sows (Sus scrofa domesticus) were divided into three treatment groups: a sentinel group (S), feedback (F), and isolated and titered virus (IV).
To evaluate the natural exposure to PEDV, 6 sows were introduced as the sentinel group (S). These animals received no PEDV prophylaxis but could have been exposed to the virus naturally at the facility by the inoculated sows. In the Feedback group (F), 14 sows were treated orally with the intestinal contents of piglets infected with PEDV. The isolated virus (IV) group was composed of 12 sows treated orally with the isolated virus with a titer of 1X108 TCID50/mL (2ml). Serum samples were taken from the sentinel, feedback, and isolated virus groups.
Feedback preparation
For the preparation of the feedback, intestinal scrapings from piglets that had been slaughtered and showed diarrhea in the last four hours were used. The proportion used was one litter per half a liter of evaporated milk, with a liquid and not pasty consistency, the presence of the virus in intestinal homogenate was confirmed by RT-qPCR on a QuantStudioTM 5 System (cat. Number: A34322, Applied Biosystems™, USA) using the VetMAXTM PEDV/TGEV/SDCoV Kit RT-qPCR assay (cat. number: A33402, Applied Biosystems™). The CT value of the chosen homogenate was 15.9 (2.8x106 copies), samples were liquified, and 2 ml were administered orally per sow.
Samples
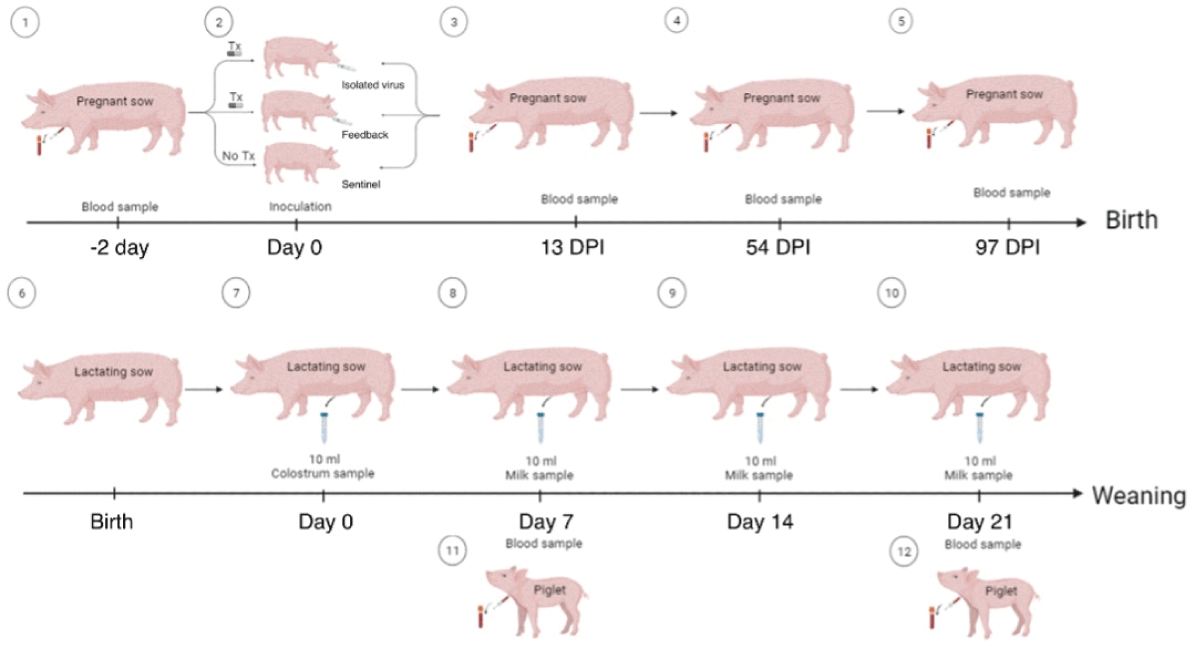
Sows: Blood samples were obtained from gestating sows from the jugular vein before and after treatment (T1: 2 days before inoculation, T2: 13 days post-inoculation, T3: 54 days post-inoculation and T4: 97 days post-inoculation). The serum obtained was stored in sterile 1.5 mL tubes at -20 °C until use. Colostrum and milk samples from lactating sows were collected every 7 days from birth until weaning (colostrum = day 0, milk = day 7, 14, and 21) as illustrated in Figure 1. Prior to collection, the mammary gland was cleaned with paper towels and benzalkonium chloride. The samples were collected into sterile 15 mL centrifuge tubes (FalconTM) and stored at -20 °C. Prior to use, the samples were thawed and centrifuged at 13,000 g for 15 minutes at 4 °C to remove fat and organic material.
Figure 1: Schematic representation of inoculation procedure and sampling. Days post-inoculation (DPI).
Blood samples from piglets were obtained from the anterior vena cava on days 7 and 21 of age. The serum was stored in sterile 1.5 mL tubes at -20 °C until use.
Enzyme-linked immunosorbent assay (ELISA) for detection of IgG
PEDV-specific IgG was detected in the serum, colostrum, and milk samples from sows and serum samples from piglets using the ELISA technique with the ID Screen® PEDV (Indirect kit ID Screen®) commercial kit, following the manufacturer’s instructions. The colostrum and milk samples were centrifuged for 15 minutes at 1000 x g at 2-8 °C, the aqueous fraction was collected and repeated twice. The relative amounts of antibodies in samples can then be calculated by reference to the positive control. This relationship is expressed as S/P ratio (Sample to Positive Ratio). An S/P% ratio ≥60% was considered antibody positive and < 60% was considered negative as recommended by the manufacturer. SP value was calculated using the following formula:
ELISA for the detection of IgA
To detect PEDV-specific IgA in the colostrum and milk of lactating sows, an ELISA test was carried out according to the report by Bjustrom, et al. 2018. The ELISA plate (Costar™ EIA/RIA, 96 wells) was sensitized with the purified virus by polyethylene glycol (PEG) precipitation and sucrose gradient in carbonate buffer (3.03 μg/mL protein per well) [30], and incubated for 16 h at 4 °C, then washed three times, with wash solution (Phosphate Buffer Solution; PBS; with Tween 20 at 0.2%). 200 µL of blocking buffer was added (PBS at 2% with skimmed milk (Nestlé) and 0.02% Triton X-100) and incubated for 1 hour at 37 °C. At the end of the incubation, the plate was washed three times with a wash solution. 100 µL of the dilutions of colostrum or milk whey were used, at a 1:10 dilution using skimmed milk (Nestlé) as the negative control. It was incubated for 1 h at 37 °C and washed three times as described above. Goat Anti-pig IgA HRP Conjugated Antibody (Bethyl laboratories) diluted at 1:500 and 100 µL of Novex® HRP Chromogenic Substrate TMB (InvitrogenTM) were used. Optical density (OD) readings were taken after 15, 30, and 45 min with a 405 nm filter in a Multiskan Ex spectrophotometer (Thermo Fisher Scientific). Values above two standard deviations of the OD of the negative control were considered positive [17].
Neutralization of viral infectivity
A total of 30,000 Vero cells were seeded per well in a flat-bottom 96 wells cell culture plate in Dulbecco′s Modified Eagle′s Medium-high glucose, supplemented with fetal bovine serum (FBS) 8% and glutamine 10 mM (propagation medium) and incubated at 37 °C in a CO2 atmosphere for 24 h.
Serum samples were treated for complement inactivation at 56 °C for 30 min then stored at -20 °C. In a 96-well plate with no cells, we placed 90 µL of D-MEM high glucose medium, supplemented with 15 µg trypsin and glutamine at 10 mM (infection medium). Double serial dilutions of samples were carried out for serum, colostrum, and milk in the infection medium. We added 100 µL of PEDV at an infection multiplicity of 0.1 to each of the wells with serum. Mock-infected cells were used as control. In parallel, wells were infected with PEDV at an infection multiplicity of 0.1 as an infection control, and wells with an infection medium were used as a cell control. All the conditions were performed in duplicate. The plate was incubated at 37 °C for 1 h. After that time, the contents were transferred from the dilution plate to the plate with cells and incubated at 37 °C in a CO2 atmosphere until cytopathic effects (CPE) were observed. Once CPE was observed, the plate was fixed for 24 h in 1% Formaldehyde in PBS and we added crystal violet in formaldehyde (37%) and incubated at 1 h at room temperature. Finally, the plate was washed under running water and the neutralizing titer was determined by TCID50/ml, as the reciprocal of the highest dilution of serum that showed neutralizing activity.
ELISA for IL-12 and IL-22
IL-12 and IL-22 were determined in the serum of gestating sows using the commercial kits Porcine IL-12 (cat. number: ESIL12A, Thermofisher®) and Porcine IL-22(IL22A) (cat. number: ES13RB, Invitrogen®), respectively, according to the manufacturer’s instructions. For quantification of IL-12 and IL-22 concentrations, a standard curve was done, and all background absorbance was subtracted from all data points, including standards, samples, and controls. Sample concentrations and controls were determined from the standard curve and value was calculated by the appropriate factor to correct for each sample dilution.
Statistical analysis
To assess the effect of treatments on the immune response in sows, measured by IgG concentration, as well as neutralization results, a Mann-Whitney U test was used [31].
To determine treatment effects on piglet, colostrum, and milk IgG, as well as IL12 and IL22 levels over time, a repeated measures model was used [32]. Differences in concentrations over time were analyzed, and the best covariance structure was determined. An adjusted Tukey’s test was applied for post-hoc comparisons [33].
Spearman’s correlation tests were performed to assess the strength of the binomial relationship: a) sow-piglets; b) mother-colostrum; and c) colostrum-pig. The unit of measurement was the concentration of IgG and these correlations were done with the direct inoculum, as the immune response is presumed to be specific to PEDV.
IgG in female and piglet serum
Our results showed that both the Feedback (F) and Isolated Virus (IV) groups seroconverted. In the F group, 66.7% of sows were seropositive, while the IV group had 76% of females with higher SP values, although there were no statistically significant differences between the two groups.
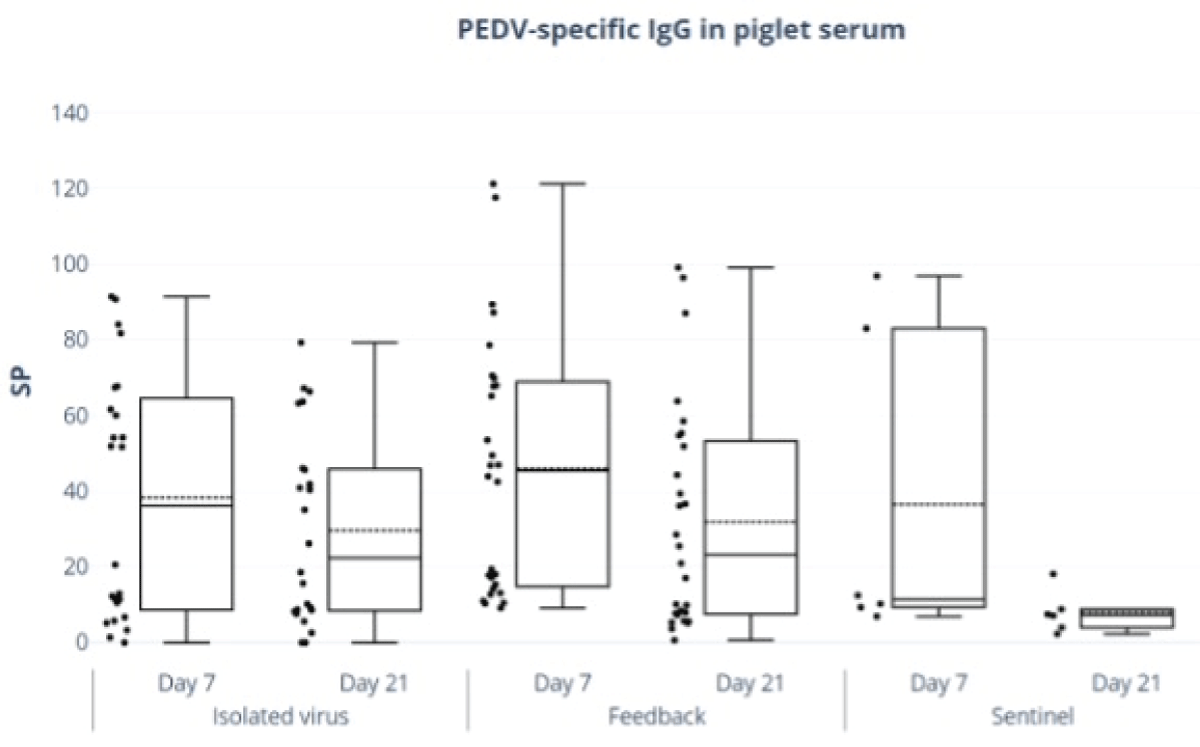
In piglets, the isolated virus group did not show a significant reduction in IgG levels from day 7 to day 21. In contrast, the feedback group exhibited a significant decrease in mean SP values by day 21 (p < 0.05), as shown in Figure 2 and Table 1.
Figure 2: Piglet serum IgG anti-PEDV antibodies. ELISA ID Screen® PEDV (Indirect kit ID Screen®) was done. Piglet serum SP values of IgG by days of age.
| Table 1: Standard Median SP values for total IgG in piglets at 7 and 21 days of age. | ||
| Treatment group | Piglet Age | |
| 7 days | 21 days | |
| Isolated Virus | 38.25± 6.21 | 29.37 ± 6.21 |
| Feedback | 46.01 ± 8.46* | 31.91 ± 8.46* |
| Sentinel | 36.50 ± 13.89* | 7.99 ± 13.89* |
| * Statistically significant difference (p < 0.05). | ||
Comparison of IgG and IgA levels in colostrum and milk by treatments
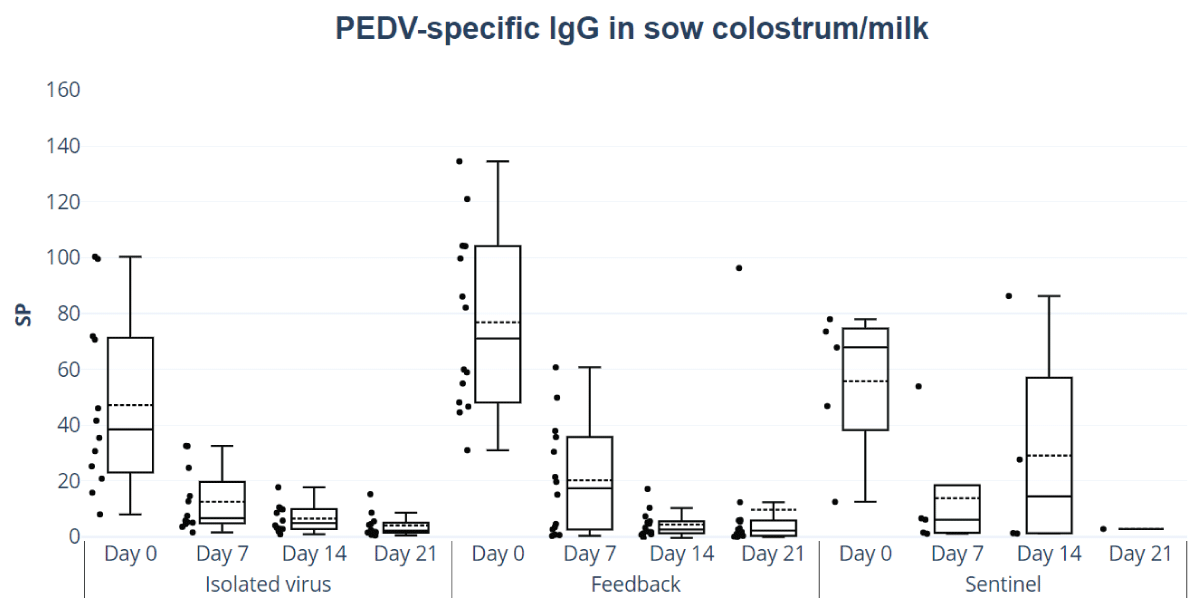
The SP values of total IgG in colostrum and milk showed no significant differences between feedback and isolated virus groups, nor did the analysis by day of lactation reveal any significant variations (Figure 3). The colostrum and milk presented a significant decrease in IgG levels over the days (p < 0.05) for all treatments.
Figure 3: Colostrum and milk IgG anti-PEDV antibodies responses. ELISA ID Screen® PEDV (Indirect kit ID Screen®). SP values of IgG in colostrum and milk, feedback, and isolated and titered virus and sentinel group treatment of IgG in colostrum and milk by day of lactation (colostrum=day 0, milk=days 7, 14, and 21).
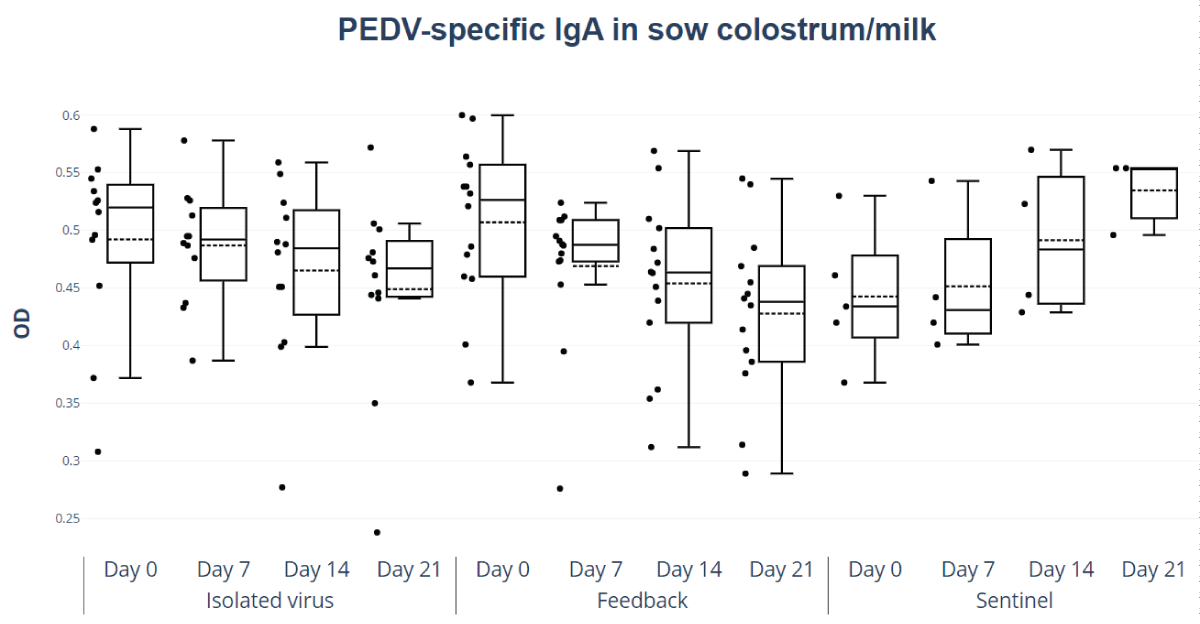
The OD values of total IgA in colostrum and milk did not show significant differences between feedback and isolated virus. However, as shown in Figure 4, both groups exhibited higher OD values on the day of birth (day 0 post-birth), which decreased on subsequent days at different rates depending on the treatment (Figure 4). Only the feedback group showed a significant reduction between days 7 and 21 (p < 0.05 and 0.043, respectively).
Figure 4: Colostrum and milk IgA PEDV antibodies responses. Optical density (OD) values of IgA in colostrum and milk, feedback, and isolated and titered virus treatment, by day of lactation (colostrum= day 0, milk=days 7, 14, and 21).
Detection of antibodies by viral neutralization
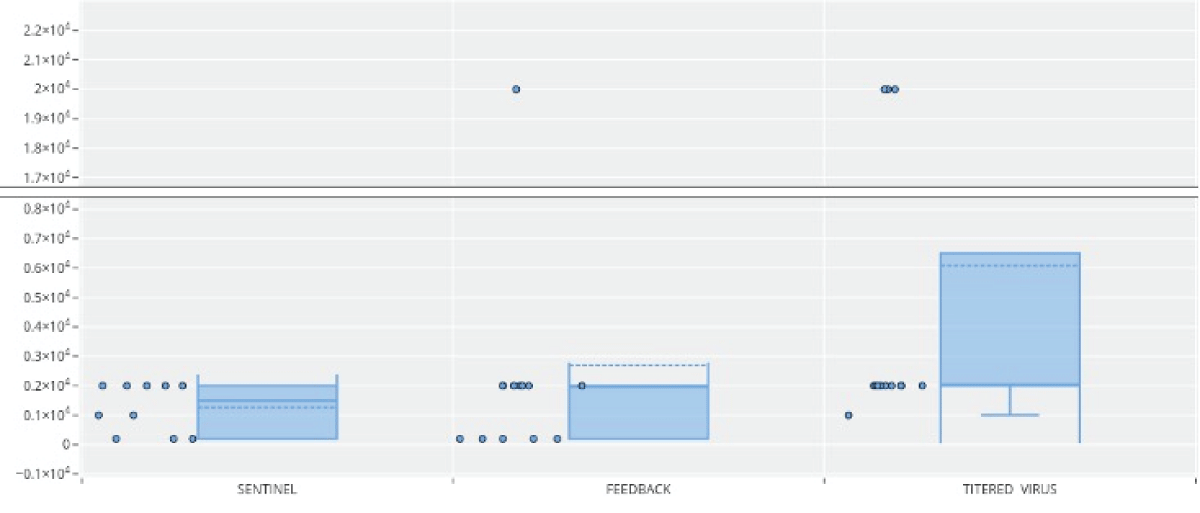
The results of the neutralizing titers by groups showed that in the sentinel group (Figure 5), 50% of the individuals had neutralizing titers of 2 x 103, while the other 50% had lower titers (1 x 102- 2 x 102). In the feedback group, 50% of the individuals had neutralizing titers of 2 x 103 and the rest had titers of 2 x 102, except for one individual who presented a value of 2 x 104. In the isolated virus group, 66% of individuals presented titers of 2 x 103; the lowest titer value was 1 x 103, and some individuals in this group had much higher titers, as shown in Figure 5. However, when comparing neutralization values, a significant difference was observed in the isolated virus group (p < 0.05) compared to the feedback and sentinel groups.
Figure 5: Porcine epidemic diarrhea virus antibody responses in sera of sows based on neutralization of viral infectivity in cell culture. Sentinel, Feedback, and Isolated and titered virus.
IL-12 and IL-22
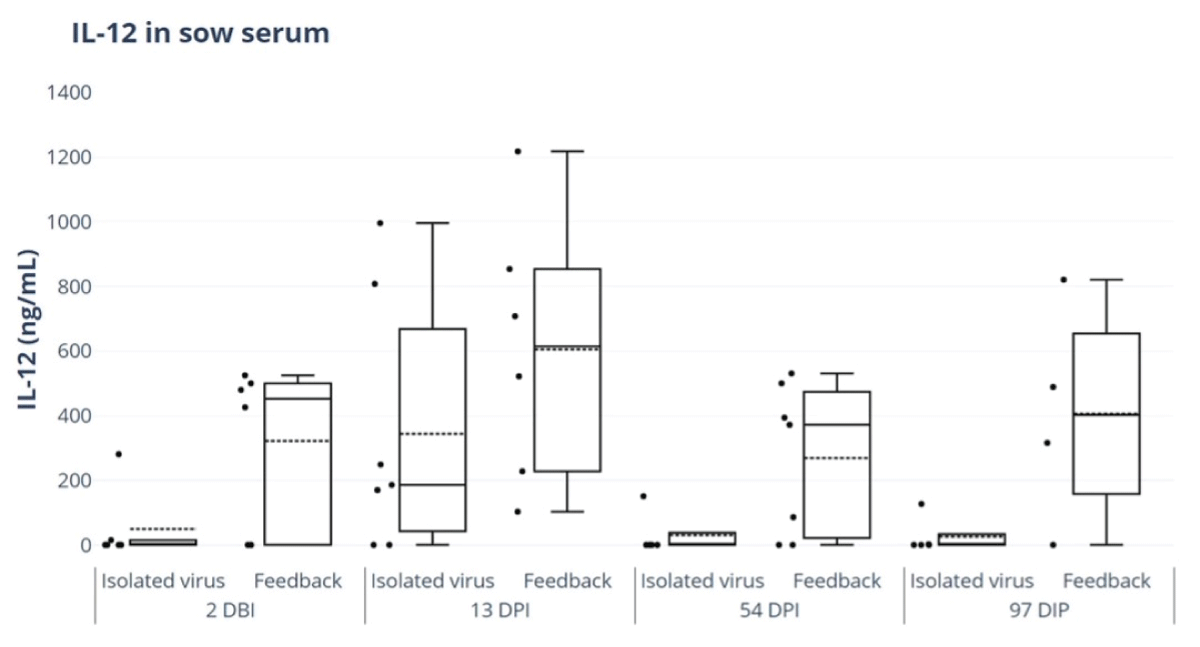
Porcine IL-22 ELISA Kit was used to evaluate IL-22 and IL-12 responses in sow serum for the feedback and isolated and titered virus treatment groups. No significant differences were detected in the levels of IL-12 or IL-22 between the different study groups; however, IL-12 levels exhibited an earlier onset and longer duration in the feedback group, where high levels were still detected on day 97, while the titered virus group no longer had detectable levels (Figure 6).
Figure 6: ELISA of IL-12 in sow serum for feedback and isolated virus treatment groups. ELISA Porcine IL-12 kit (IL12A) (Thermofisher®). 2 days before inoculation (DBI), 13 days post-inoculation (DPI), 54 DPI and 97 DPI.
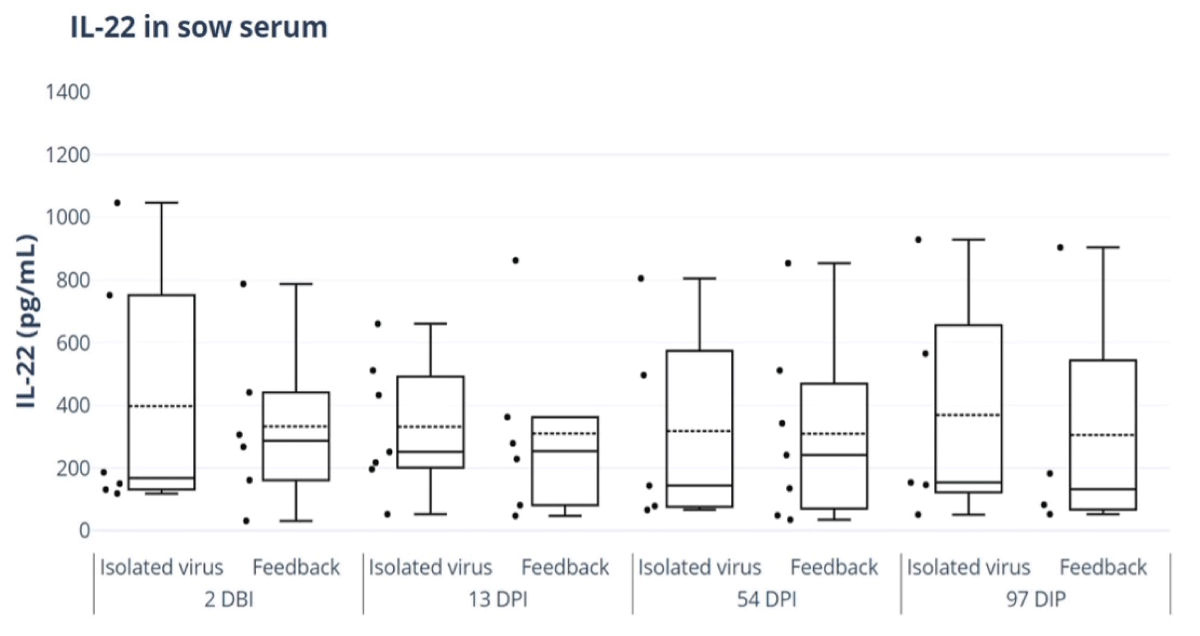
The duration of the IL-22 response was not significantly different between treatments and was present in both groups until 97 DPI (Figure 7).
Figure 7: ELISA of IL-22 responses in sow serum for feedback and isolated virus treatment groups. ELISA Porcine IL-22 kit (IL22A) (Thermofisher®). 2 days before inoculation (DBI), 13 days post-inoculation (DPI), 54 DPI and 97 DPI.
Productive parameters
In this study, we measured the total number of born piglets per litter, stillborn piglets per litter, mummified piglets, litter weight at birth, weaned piglet yield, number of dead piglets during lactation, and weight per litter at weaning, there were no significant statistical differences (p > 0.05) between the study groups. Additionally, no significant differences were observed in female weaning performance, fertility at calving, or the number of days to return to estrus post-weaning.
The appearance of PEDV in the Americas beginning in 2013 had a significant impact on porcine health due to the lack of vaccines and specific treatments. The control measures proposed were based on little-evaluated techniques and continue to be used today because of their low cost. In Mexico, PEDV vaccines have been used for control since 2018; however, the efficacy of existing vaccines often fails in endemic settings and naïve herds. One reason for this variability in vaccine efficacy under field conditions may be that parenteral inactivated vaccines induce weaker and less persistent lactogenic immunity compared to the oral administration of live virus vaccines, as described by Opriessnig, et al. 2017 [34]. Additionally, the use of attenuated vaccines or feedback treatment could increase the genetic interactions of viruses; these interactions can occur both with vaccines made from attenuated viruses and with feedback treatment. For this reason, constant monitoring of circulating viral strains on farms is crucial, and good farm management practices, including vaccination and herd monitoring, are essential to control the spread of PEDV and manage the impact of recombinant strains. Since suckling neonates are the population most susceptible to PEDV infection, maternal vaccination is an integral strategy for passive lactogenic protection to prevent and eradicate PED outbreaks, whether epidemic or endemic.
Because of the impermeable placenta, piglets are born with no gamma globulins and are highly susceptible to many infectious agents. As a result, protection from infections is based exclusively on colostrum and milk antibodies. Identifying the factors that influence lactogenic immunity and the intestinal-mammary-secretory axis could lead to better control strategies for PEDV and other enteric pathogens and improve swine health and industry productivity. The principles of passive immunity for TGEV, rotavirus, and PEDV are largely the same. In each case, passive immunity is provided through colostrum, where antibodies (especially IgA) offer protection against enteric infections, but the effectiveness can depend on factors like the sow´s immune status and the specific characteristics of the virus in questions, vaccination of sows can improve the level of protection passed on to piglets.
Feedback treatment has been used to control enteric viruses; however, it presents potential risks due to uncontrolled fluctuations in viral load administration. Since the treatment is not consistently uniform, it could result in reinfection of pigs with varying viral doses, affecting the severity of the disease and the accuracy of disease modeling. This variability can cause unpredictable disease progression and complicate efforts to control the spread of the virus within a farm. Inconsistent treatment protocols can lead to unpredictable results, making it difficult to determine the success of the approach in different settings, especially when the methodology is not standardized. Moreover, enteric viruses such as PEDV, TGEV, rotavirus, and others often weaken the immune system of pigs, leaving them more susceptible to secondary bacterial infections. Feedback treatment may not account for the presence of other pathogens in the environment, leading to a higher risk of complications or co-infections, increased mortality rates, and reduced effectiveness. The feedback treatment also raises biosecurity concerns, as there is a high risk of contaminating equipment, workers, and facilities. Poor biosecurity practices during the feedback process can facilitate the spread of the virus to other farms or pig populations, complicating eradication efforts and increasing the overall cost of managing the outbreak.
The current study, which compares the effects of inoculation using feedback versus isolated and titered PEDV, found no difference in anti-PEDV IgG levels in sows or piglets between both treatments. This is consistent with findings by Wang, et al. 2019 in mice and of Krishna, et al. 2020 in swine, where the immunized groups did not present significant differences in the first days after treatment [35,36]. Even when analyzing IgG in piglets, separately by sex (females and castrated males), there was still no statistically significant difference among groups, and there were significant differences in IgG as a function of piglet age, independent of treatment (7 and 21 days). These results could be due to the limited absorption of IgG antibodies over the days [37]. Other authors have reported similar results to our study, showing concordance in antibody levels in serum and mammary secretions of sows and in piglet serum [38].
Our results showed that both the feedback and isolated virus groups seroconverted. However, there was a difference related to treatment type, with the isolated virus group showing higher values than the feedback group. Specifically, 66.7% of sows in the feedback group were seropositive, while 76% of females in the isolated virus group had higher SP values, indicating a greater presence of antibodies. Since lactogenic immunity protects suckling piglets from PEDV infection, the induction of immune responses in females may be an effective means of protection, this protection is related to the inoculation dose and is enhanced with increasing concentrations. Therefore, studies with varying virus concentrations are necessary to better understand the role of inoculum concentration. Our results are consistent with the report by Bertasio 2016, et al. which found an average seroconversion rate of 61.6% following an evaluation of farrow-to-finish farms in Italy; that study also highlighted the importance of maintaining seropositive sows as a strategy to prevent severe cases of PED in neonates [39].
The levels of IgA and IgG in colostrum and milk showed a significant 32% association between IgA levels and days of lactation. In contrast, there was no association between the number of births and IgA levels, and the results were similar for IgG levels. There were no significant statistical differences (p > 0.05) between the study groups in terms of total number of piglets born per litter, stillborn piglets per litter, mummified piglets, litter weight at birth, weaned piglet yield, piglets dying during lactation, and weight per litter at weaning. Furthermore, no statistically significant differences were found in female weaning performance, fertility at calving, or days to return to estrus post-weaning. These findings are consistent with the studies by Furutani, et al. 2017, 2018, and 2019. This suggests that the recovery of pork production parameters depends on multiple factors, including the scale of production at the time of evaluation [40-43].
Although no significant differences related to treatment were found, difficulties in controlling the viral load (infectious virus), particularly in the early stages, have the potential to exacerbate the damage caused by PEDs. Therefore, the use of isolated viruses prevents the spread of other diseases and allows for better control of the infectious virus in the inoculum. Additionally, early detection may help mitigate the damage caused by outbreaks.
The levels of IgG and IgA immunoglobulins in mammary secretions did not differ among the treatment groups. However, a significant drop (p < 0.05) in IgG levels was observed in all treatments. Differences in IgG and IgA immunoglobulins in mammary secretions were only detected when using the direct inoculum on the day of birth.
Under normal conditions, the antibody composition in mammary secretions varies throughout the course of lactation. During the colostrum phase, which occurs shortly after birth, there is a higher concentration of IgG. The concentration of immunoglobulins begins to decrease 6 hours postpartum, with a reduction of 30% to 45% by 12 hours [37,44]. This decrease continues over the next 24 to 48 hours, during which colostrum transitions into milk, in which IgA is found in higher proportions. In our present study, there were significant differences in immunoglobulin levels on different sampling days, with day 0 showing the highest levels and a gradual decrease over the course of the days.
These results coincide with those of the studies by Carney-Hinkle, et al. 2013, Srijangwad, et al. 2017, Joshi, et al. 2018 and Jung, et al. 2020, where the highest immunoglobulin contents occurred during the first days of lactation [14,45-47]. The results from the group of sows inoculated during the last third of gestation showed that on day 0, the levels of immunoglobulins were higher than on days 15 and 22, regardless of treatment. This contrasts with the report by Langel, et al. 2019 [48], where immunoglobulin levels decreased from day 0 to days 3–5, then increased from days 8–14 and 15–22 in sows inoculated during the first and second thirds of gestation, respectively. However, it must be considered that all sows were from the first parity. Further studies must be conducted to analyze different parity numbers, types of inoculums, and frequencies of treatment [45-47-,49].
The presence of antibodies in colostrum and milk does not necessarily indicate that they can neutralize the virus and protect the piglet. There was no difference in the concentration of IgG related to treatment type in the serum of the mothers. However, when comparing neutralization values, a significant increase was observed when an isolated virus was used (p < 0.05) compared to feedback. Due to the high virulence of PEDV and the immature and naïve immune system of neonatal suckling piglets, passive lactogenic immunity to PEDV induced during gestation is critical for piglet protection. Piglet survival positively correlated with PEDV IgA and neutralizing antibodies in milk and PEDV IgA and IgG antibodies in piglet serum. A treatment that produces neutralizing antibodies will provide the protection necessary for piglet survival in the face of any challenge. On the other hand, when immunized with pathogenic strains of PEDV, there was an increase in neutralizing antibodies [36,38,50]. This suggests that the differences observed may be determined by the inoculum, as was observed in our study.
Finally, we analyzed the concentrations of IL-12 and IL-22 in response to the different treatments. One cytokine is involved in maintaining mucosal integrity (IL-12), while the other inhibits PEDV in vitro (IL-22). IL-12 was more activated in response to feedback, but we also observed an IL-12 response to the isolated titered virus, suggesting that this cytokine may help maintain intestinal integrity in infected animals. This result can be explained by the composition of the feedback treatment, as it contains not only PEDV, but also other viruses, bacteria, and parasites present on the farm. It has been reported that recombinant IL-22 inhibits the replication of PEDV in vitro [28,51] and it also inhibits TGEV and porcine rotavirus. The mechanism of antiviral action of IL-22 is still unknown. It is critical to continue studying the immune response to better understand the pathogenesis of PEDV infection.
Given the significant economic losses caused by PEDV and the difficulty in obtaining effective vaccines in some regions, an efficient alternative control method is becoming increasingly important. We compared the efficacy of two types of immunogenic material in terms of IgG, IgA, neutralizing antibodies, and two cytokines in our study. More research is needed to determine the mechanism and role of IL-12 in swine intestinal barrier protection, as well as its relevance in PEDV infections. Simultaneously, we demonstrated that the viral inoculum could elicit a lactogenic immune response with both IgA and IgG while preventing the spread of other pathogens. Our findings are in concordance with reports by Choe, et al. 2020, who propose the use of an orally administered vaccine to induce protective lactogenic immunity [52].
In this study, the productive parameters analyzed included the total number of piglets born, mortality at weaning, average birth weight, and the number of stillborn piglets. Mortality at weaning and the number of stillborn piglets were higher in animals treated with Feedback (48.31% and 20.96%, respectively) compared to those that received the isolated virus (30.02% and 10.44%, respectively). Therefore, the isolated virus treatment could offer a more secure, long-lasting, and specific immune response.
Maternal antibodies provide the necessary lactogenic immunity required for neonatal piglet immune defense until endogenous antibodies can be produced in sufficient amounts. However, various factors can influence the quantity and quality of secreted antibodies, such as the stage of gestation, sow age (parity), hormones, body condition, and the timing and route of inoculation. Given the low efficiency of currently available vaccines, the use of a viral inoculum that is isolated and titrated can provide long-lasting, specific, and neutralizing antibodies. This approach also offers a safe method of protection for the piglets, as it prevents the spread of other diseases and helps reduce both productive and economic losses. Evaluating long-term immunity after treatments such as feedback, isolated virus inoculation, or even vaccines is crucial for maintaining immunity in breeding herds. A study focused on the persistence of immune responses (e.g., serum antibodies and mucosal immunity) over extended periods would help clarify the protective effects of these treatments. The cost-effectiveness of the approach would depend on the size of the facility. Future studies could explore how factors such as biosecurity measures, environmental control, and nutritional strategies influence the effectiveness of PEDV treatments, which could be essential for optimizing vaccination programs and ensuring the best possible outcomes in the field.
Maternal antibodies provide the necessary lactogenic immunity needed for neonatal piglet immune defense until endogenous antibodies can be produced in sufficient amounts, however, various factors can influence the quantity and quality of secreted antibodies such as gestation stage, sow age (parity), hormones, body condition, time and route of inoculation. Given the low efficiency of currently available vaccines, the use of a viral inoculum that is isolated and titrated can provide long-lasting, specific, and neutralizing antibodies. This approach is also a safe method of protection for the piglets, as it prevents the spread of other diseases and helps reduce productive and economic losses.
Funding statement
This research was funded by Universidad Nacional Autónoma de México (www.unam.mx), grant number PAPIIT IN222020, awarded to Sarmiento-Silva RE. CONAHCyT by scholarship from Fernández and García. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or ϐinancial relationships that could be construed as a potential conϐlict of interest.
Author contributions
Conceptualization. RE. Sarmiento-Silva, ME Trujillo-Ortega
Data curation: F. Martínez-Castañeda, ME. Garcia-Hernandez, Soϐía-Lizeth Alcaraz-Estrada
Formal analysis: S. Fernández-Hernández
Funding acquisition: RE. Sarmiento-Silva
Investigation: RE. Sarmiento-Silva, ME Trujillo-Ortega
Methodology: S. Fernández-Hernández, R. Beltrán-Figueroa, C. Itzel Vergara-Zermeño Elein Hernandez-Trujillo
Resources: RE. Sarmiento-Silva, ME Trujillo-Ortega
Project administration: RE. Sarmiento-Silva
Writing–original draft: S. Fernández-Hernández, ME. García-Hernández, RE. Sarmiento-Silva
Writing–review and editing: S. Fernández-Hernández, ME. García-Hernández, RE. Sarmiento-Silva
The authors would like to thank MC. Elvia Lazo García, and MVZ Ma. Grisel Anaya Santillán for their support in the laboratory work, MVZ MC. Víctor Martínez Torres and MVZ PhD Miguel González Lozano for their support in the pig farm and Lynna Kiere for English revisions. Thanks go to the Post Graduate program in Ciencias de la Producción y de la Salud Animal and CONAHCyt for the scholarship received. Dirección General del Personal Académico, UNAM. For the RESS sabbatical fellowship at the International Joint Laboratory ELDORADO, (DGAPA, PASPA 2021-2022).
- Duarte M, Gelfi J, Lambert P, Rasschaert D, Laude H. Genome organization of porcine epidemic diarrhoea virus. Adv Exp Med Biol. 1994;342:55-60. Available from: https://doi.org/10.1007/978-1-4615-2996-5_9
- Kocherhans R, Bridgen A, Ackermann M, Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23(2):137-44. Available from: https://doi.org/10.1023/a:1011831902219.
- Song D, Moon H, Kang B. Porcine epidemic diarrhea: a review of current epidemiology and available vaccines. Clin Exp Vaccine Res. 2015;4(2):166-76. Available from: https://doi.org/10.7774/cevr.2015.4.2.166
- Pensaert MB, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58(3):243-247. Available from: https://doi.org/10.1007/BF01317606.
- Trujillo-Ortega ME, Beltrán-Figueroa R, García-Hernández ME, Juárez-Ramírez M, Sotomayor-González A, Hernández-Villegas EN, et al. Isolation and characterization of porcine epidemic diarrhea virus associated with the 2014 disease outbreak in Mexico: case report. BMC Vet Res. 2016; 12: 132. Available from: https://doi.org/10.1186/s12917-016-0763-z.
- Wang D, Fang L, Xiao S. Porcine epidemic diarrhea in China. Virus Res. 2016; 226: 7-13. Available from: https://doi.org/10.1016/j.virusres.2016.05.026.
- Schwartz TJ, Rademacher CJ, Gimenez-Lirola LG, Sun Y, Zimmerman JJ. Evaluation of the effects of PEDV vaccine on PEDV naïve and previously PEDV-exposed sows in a challenge model comparing immune response and preweaning mortality. In: McKean JD, editor. Swine Disease Conference Proceedings. Ames, IA: Iowa State University; 2015. p. 36-40.
- Lee SH, Yang DK, Kim HH, Cho IS. Efficacy of inactivated variant porcine epidemic diarrhea virus vaccines in growing pigs. Clin Exp Vaccine Res. 2018; 7(1): 61-9. Available from: https://doi.org/10.7774/cevr.2018.7.1.61.
- Song DS, Oh JS, Kang BK, Yang JS, Moon HJ, Yoo HS, et al. Oral efficacy of Vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Res Vet Sci. 2007; 82(2): 134-40. Available from: https://doi.org/10.1016/j.rvsc.2006.03.007.
- Geiger JO, Connor JF. Porcine Epidemic Diarrhea, Diagnosis and Elimination. In: American Association of Swine Veterinarians; 2013. p. 1-4.
- Lin C-M, Saif LJ, Marthaler D, Wang Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016; 226: 20-39. Available from: https://doi.org/10.1016/j.virusres.2016.05.023.
- Li ZL, Zhu L, Ma JY, Zhou QF, Song YH, Sun BL, et al. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in south China. Virus Genes. 2012. Available from: https://doi.org/10.1007/s11262-012-0735-8.
- Sun J, Li Q, Shao C, Ma Y, He H, Jiang S, et al. Isolation and characterization of Chinese porcine epidemic diarrhea virus with novel mutations and deletions in the S gene. Vet Microbiol. 2018; 221: 81-9. Available from: https://doi.org/10.1016/j.vetmic.2018.05.021.
- Jung K, Saif LJ, Wang Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020; 286: 198045. Available from: https://doi.org/10.1016/j.virusres.2020.198045.
- Lee C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol J. 2015; 12: 193. Available from: https://doi.org/10.1186/s12985-015-0421-2.
- Bohl EH, Gupta RK, Olquin MV, Saif LJ. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972; 6(3): 289-301. Available from: https://doi.org/10.1128/iai.6.3.289-301.1972.
- Bjustrom-Kraft J, Woodard K, Giménez-Lirola L, Setness B, Ji J, Lasley P, et al. Serum and mammary secretion antibody responses in porcine epidemic diarrhea-immune gilts following porcine epidemic diarrhea vaccination. J Swine Health Prod. 2018; 26: 34-40.
- Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol. 2009; 33(4): 384-93. Available from: https://doi.org/10.1016/j.dci.2008.07.007.
- Scherba G, Bromfield CR, Jarrell VL, Shipley CF. Evaluation of responses to both oral and parenteral immunization modalities for porcine epidemic diarrhea virus in production units. J Swine Health Prod. 2016; 24: 29-35.
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003; 3(2): 133-46. Available from: https://doi.org/10.1038/nri1001.
- Vignali DAA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012; 13(8): 722-8. Available from: https://doi.org/10.1038/ni.2366.
- Behzadi P, Behzadi E, Ranjbar R. IL-12 family cytokines: General characteristics, pathogenic microorganisms, receptors, and signalling pathways. Acta Microbiol Immunol Hung. 2016; 63(1): 1-25. Available from: https://doi.org/10.1556/030.63.2016.1.1.
- Saeng-chuto K, Madapong A, Kaeoket K, Piñeyro PE, Tantituvanont A, Nilubol D. Coinfection of porcine deltacoronavirus and porcine epidemic diarrhea virus increases disease severity, cell trophism and earlier upregulation of IFN-α and IL12. Sci Rep. 2021; 11: 3040. Available from: https://doi.org/10.1038/s41598-021-82738-8.
- Keir ME, Yi T, Lu TT, Ghilardi N. The role of IL-22 in intestinal health and disease. J Exp Med. 2020; 217: e20192195. Available from: https://doi.org/10.1084/jem_20192195.
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008; 14(3): 282-9. Available from: https://doi.org/10.1038/nm1720.
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011; 12(4): 383-90. Available from: https://doi.org/10.1038/ni.2025.
- Li Y, Wang J, Li Y, Wu H, Zhao S, Yu Q. Protecting intestinal epithelial cells against deoxynivalenol and E. coli damage by recombinant porcine IL-22. Vet Microbiol. 2019; 231: 154-9. Available from: https://doi.org/10.1016/j.vetmic.2019.02.027.
- Xue M, Zhao J, Ying L, Fu F, Li L, Ma Y, et al. IL-22 suppresses the infection of porcine enteric coronaviruses and rotavirus by activating STAT3 signal pathway. Antiviral Res. 2017; 142: 68-75. Available from: https://doi.org/10.1016/j.antiviral.2017.03.006.
- García-Hernández M-E, Trujillo-Ortega M-E, Alcaraz-Estrada S-L, Lozano-Aguirre-Beltrán L, Sandoval-Jaime C, Taboada-Ramírez BI, et al. Molecular Detection and Characterization of Porcine Epidemic Diarrhea Virus and Porcine Aichivirus C Coinfection in México. Viruses. 2021; 13(5): 738. Available from: https://doi.org/10.3390/v13050738.
- Sarmiento RE, Tirado R, Gómez B. Characteristics of a respiratory syncytial virus persistently infected macrophage-like culture. Virus Res. 2002; 84(1-2): 45-58. Available from: https://doi.org/10.1016/S0168-1702(01)00420-8.
- Higgins J. An introduction to non-parametric statistics. Anal Methods. 2013; 5(19): 5373-4. Available from: https://doi.org/10.1039/c3ay90070c.
- Park E, Cho M, Ki CS. Correct use of repeated measures analysis of variance. Korean J Lab Med. 2009; 29(1): 1-9. Available from: https://doi.org/10.3343/kjlm.2009.29.1.1.
- Kramer CY. Extension of Multiple Range Tests to Group Correlated Adjusted Means. Biometrics. 1957; 13: 13. Available from: https://doi.org/10.2307/3001898.
- Opriessnig T, Gerber PF, Shen H, de Castro AMMG, Zhang J, Chen Q, et al. Evaluation of the efficacy of a commercial inactivated genogroup 2b-based porcine epidemic diarrhea virus (PEDV) vaccine and experimental live genogroup 1b exposure against 2b challenge. Vet Res. 2017; 48: 69. Available from: https://doi.org/10.1186/s13567-017-0472-z.
- Wang Q, Vlasova AN, Kenney SP, Saif LJ. Emerging and re-emerging coronaviruses in pigs. Curr Opin Virol. 2019; 34: 39-49. Available from: https://doi.org/10.1016/j.coviro.2018.12.001.
- Krishna VD, Kim Y, Yang M, Vannucci F, Molitor T, Torremorell M, et al. Immune responses to porcine epidemic diarrhea virus (PEDV) in swine and protection against subsequent infection. PLoS One. 2020; 15(4): e0231723. Available from: https://doi.org/10.1371/journal.pone.0231723.
- Poonsuk K, Zimmerman J. Historical and contemporary aspects of maternal immunity in swine. Anim Health Res Rev. 2018; 19(1): 31-45. Available from: https://doi.org/10.1017/S1466252317000123.
- Langel SN, Wang Q, Vlasova AN, Saif LJ. Host factors affecting generation of immunity against porcine epidemic diarrhea virus in pregnant and lactating swine and passive protection of neonates. Pathogens. 2020; 9(2): 130. Available from: https://doi.org/10.3390/pathogens9020130.
- Bertasio C, Giacomini E, Lazzaro M, Perulli S, Papetti A, Lavazza A, et al. Porcine Epidemic Diarrhea Virus Shedding and Antibody Response in Swine Farms: A Longitudinal Study. Front Microbiol. 2016; 7: 2009. Available from: https://doi.org/10.3389/fmicb.2016.02009.
- Furutani A, Kawabata T, Sueyoshi M, Sasaki Y. Impact of porcine epidemic diarrhea on herd and individual Berkshire sow productivity. Anim Reprod Sci. 2017; 183: 1-8. Available from: https://doi.org/10.1016/j.anireprosci.2017.06.013.
- Furutani A, Kawabata T, Sueyoshi M, Sasaki Y. Assessment of reproductive performance in F1 sows exposed to the porcine epidemic diarrhea virus at different periods of production stage on farms with different hygienic environments. Anim Reprod Sci. 2018; 192: 233-41. Available from: https://doi.org/10.1016/j.anireprosci.2018.03.017.
- Furutani A, Sekiguchi S, Sueyoshi M, Sasaki Y. Effect of intervention practices to control the porcine epidemic diarrhea (PED) outbreak during the first epidemic year (2013–2014) on time to absence of clinical signs and the number of dead piglets per sow in Japan. Prev Vet Med. 2019; 169: 104710. Available from: https://doi.org/10.1016/j.prevetmed.2019.104710.
- Boonsoongnern P, Boodde O, Chumsing W, Sukmak M, Jirawattanapong P, Ratanavanichrojn N, et al. Correlation between antibody response against porcine epidemic diarrhea virus in sows and their offspring under field conditions. Vet World. 2021; 14(8): 1689-94. Available from: https://doi.org/10.14202/vetworld.2021.1689-1694.
- Theil PK, Hurley WL. The Protein Component of Sow Colostrum and Milk. In: Milk Proteins - From Structure to Biological Properties and Health Aspects. InTech; 2016. Available from: https://doi.org/10.5772/62841.
- Carney-Hinkle EE, Tran H, Bundy JW, Moreno R, Miller PS, Burkey TE. Effect of dam parity on litter performance, transfer of passive immunity, and progeny microbial ecology. J Anim Sci. 2013; 91(6): 2885-93. Available from: https://doi.org/10.2527/jas.2011-4874.
- Srijangwad A, Stott CJ, Temeeyasen G, Senasuthum R, Chongcharoen W, Tantituvanont A, et al. Immune response of gilts to single and double infection with porcine epidemic diarrhea virus. Arch Virol. 2017; 162(7): 2029-34. Available from: https://doi.org/10.1007/s00705-017-3307-3.
- Joshi LR, Okda FA, Singrey A, Maggioli MF, Faccin TC, Fernandes MHV, et al. Passive immunity to porcine epidemic diarrhea virus following immunization of pregnant gilts with a recombinant orf virus vector expressing the spike protein. Arch Virol. 2018; 163(9): 2327-35. Available from: https://doi.org/10.1007/s00705-018-3855-1.
- Langel SN, Paim FC, Alhamo MA, Buckley A, Van Geelen A, Lager KM, et al. Stage of Gestation at Porcine Epidemic Diarrhea Virus Infection of Pregnant Swine Impacts Maternal Immunity and Lactogenic Immune Protection of Neonatal Suckling Piglets. Front Immunol. 2019; 10: 727. Available from: https://doi.org/10.3389/fimmu.2019.00727.
- Lin C-M, Ghimire S, Hou Y, Boley P, Langel SN, Vlasova AN, et al. Pathogenicity and immunogenicity of attenuated porcine epidemic diarrhea virus PC22A strain in conventional weaned pigs. BMC Vet Res. 2019; 15: 26. Available from: https://doi.org/10.1186/s12917-018-1756-x.
- Turlewicz-Podbielska H, Pomorska-Mól M. Porcine Coronaviruses: Overview of the State of the Art. Virol Sin. 2021; 36(5): 833-51. Available from: https://doi.org/10.1007/s12250-021-00364-0.
- Li L, Fu F, Xue M, Chen W, Liu J, Shi H, et al. IFN-lambda preferably inhibits PEDV infection of porcine intestinal epithelial cells compared with IFN-alpha. Antiviral Res. 2017; 140: 76-82. Available from: https://doi.org/10.1016/j.antiviral.2017.01.012.
- Choe SE, Song S, Piao D, Park GN, Shin J, Choi YJ, et al. Efficacy of orally administered porcine epidemic diarrhea vaccine-loaded hydroxypropyl methylcellulose phthalate microspheres and RANKL-secreting L. lactis. Vet Microbiol. 2020; 242: 108604. Available from: https://doi.org/10.1016/j.vetmic.2020.108604.